Page 541 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 541
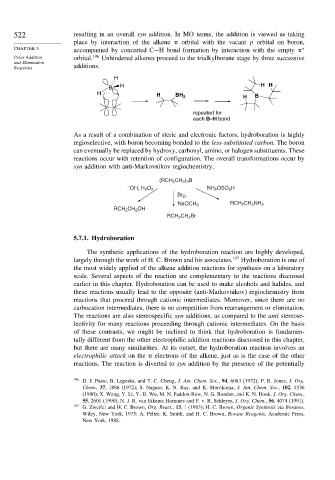
522 resulting in an overall syn addition. In MO terms, the addition is viewed as taking
place by interaction of the alkene orbital with the vacant p orbital on boron,
CHAPTER 5 accompanied by concerted C−H bond formation by interaction with the empty ∗
Polar Addition orbital. 196 Unhindered alkenes proceed to the trialkylborane stage by three successive
and Elimination
Reactions additions.
H
B H H H
H H BH 2 H B
repeated for
each B–H bond
As a result of a combination of steric and electronic factors, hydroboration is highly
regioselective, with boron becoming bonded to the less-substituted carbon. The boron
can eventually be replaced by hydroxy, carbonyl, amino, or halogen substituents. These
reactions occur with retention of configuration. The overall transformations occur by
syn addition with anti-Markovnikov regiochemistry.
(RCH 2 CH 2 ) 3 B
– NH 2 OSO 3 H
OH, H 2 O 2
Br 2 ,
NaOCH 3 RCH 2 CH 2 NH 2
RCH 2 CH 2 OH
RCH 2 CH 2 Br
5.7.1. Hydroboration
The synthetic applications of the hydroboration reaction are highly developed,
largely through the work of H. C. Brown and his associates. 197 Hydroboration is one of
the most widely applied of the alkene addition reactions for synthesis on a laboratory
scale. Several aspects of the reaction are complementary to the reactions discussed
earlier in this chapter. Hydroboration can be used to make alcohols and halides, and
these reactions usually lead to the opposite (anti-Markovnikov) regiochemistry from
reactions that proceed through cationic intermediates. Moreover, since there are no
carbocation intermediates, there is no competition from rearrangement or elimination.
The reactions are also stereospecific syn additions, as compared to the anti stereose-
lectivity for many reactions proceeding through cationic intermediates. On the basis
of these contrasts, we might be inclined to think that hydroboration is fundamen-
tally different from the other electrophilic addition reactions discussed in this chapter,
but there are many similarities. At its outset, the hydroboration reaction involves an
electrophilic attack on the electrons of the alkene, just as is the case of the other
reactions. The reaction is diverted to syn addition by the presence of the potentially
196 D. J. Pasto, B. Lepeska, and T.-C. Cheng, J. Am. Chem. Soc., 94, 6083 (1972); P. R. Jones, J. Org.
Chem., 37, 1886 (1972); S. Nagase, K. N. Ray, and K. Morokuma, J. Am. Chem. Soc., 102, 4536
(1980); X. Wang, Y. Li, Y.-D. Wu, M. N. Paddon-Row, N. G. Rondan, and K. N. Houk, J. Org. Chem.,
55, 2601 (1990); N. J. R. van Eikema Hommes and P. v. R. Schleyer, J. Org. Chem., 56, 4074 (1991).
197
G. Zweifel and H. C. Brown, Org. React., 13, 1 (1963); H. C. Brown, Organic Synthesis via Boranes,
Wiley, New York, 1975; A. Pelter, K. Smith, and H. C. Brown, Borane Reagents, Academic Press,
New York, 1988.