Page 601 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 601
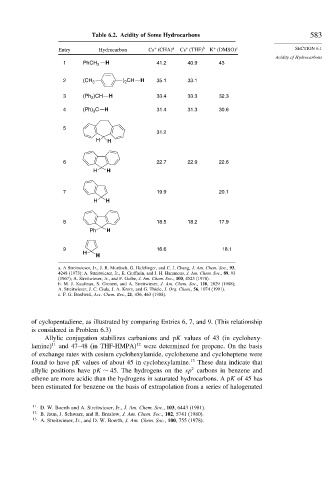
Table 6.2. Acidity of Some Hydrocarbons 583
+
+
+
Entry Hydrocarbon Cs (CHA) a Cs (THF) b K (DMSO) c SECTION 6.1
Acidity of Hydrocarbons
1 PhCH 2 H 41.2 40.9 43
2 (CH 3 ) CH H 35.1 33.1
2
3 (Ph )CH H 33.4 33.3 32.3
2
4 (Ph) C H 31.4 31.3 30.6
3
5
31.2
H H
6 22.7 22.9 22.6
H H
7 19.9 20.1
H H
8 18.5 18.2 17.9
Ph H
9 16.6 18.1
H
H
a. A Streitwieser, Jr., J. R. Murdoch, G. Hafelinger, and C. J. Chang, J. Am. Chem. Soc., 93,
4248 (1973); A. Streitwieser, Jr., E. Ciuffarin, and J. H. Hammons, J. Am. Chem. Soc., 89,93
(1967); A. Streitwieser, Jr., and F. Guibe, J. Am. Chem. Soc., 100, 4523 (1978).
b. M. J. Kaufman, S. Gronert, and A. Streitwieser, J. Am. Chem. Soc., 110, 2829 (1988);
A. Streitwieser, J. C. Ciula, J. A. Krom, and G. Thiele, J. Org. Chem., 56, 1074 (1991).
c. F. G. Bordwell, Acc. Chem. Res., 21, 456, 463 (1988).
of cyclopentadiene, as illustrated by comparing Entries 6, 7, and 9. (This relationship
is considered in Problem 6.3)
Allylic conjugation stabilizes carbanions and pK values of 43 (in cyclohexy-
lamine) 11 and 47–48 (in THF-HMPA) 12 were determined for propene. On the basis
of exchange rates with cesium cyclohexylamide, cyclohexene and cycloheptene were
found to have pK values of about 45 in cyclohexylamine. 13 These data indicate that
2
allylic positions have pK ∼ 45. The hydrogens on the sp carbons in benzene and
ethene are more acidic than the hydrogens in saturated hydrocarbons. A pK of 45 has
been estimated for benzene on the basis of extrapolation from a series of halogenated
11
D. W. Boerth and A. Streitwieser, Jr., J. Am. Chem. Soc., 103, 6443 (1981).
12 B. Jaun, J. Schwarz, and R. Breslow, J. Am. Chem. Soc., 102, 5741 (1980).
13
A. Streitwieser, Jr., and D. W. Boerth, J. Am. Chem. Soc., 100, 755 (1978).