Page 605 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 605
1 allows the stereochemical features of the anion to be probed by measuring the 587
enantiomeric purity of the 2-phenylbutane product.
SECTION 6.1
H Acidity of Hydrocarbons
3
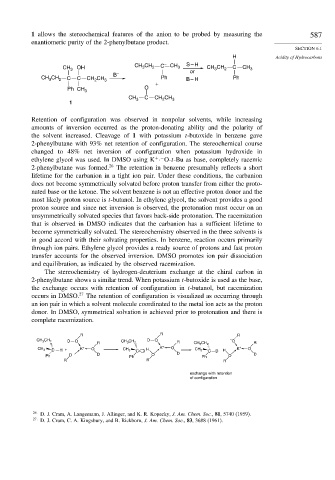
CH 3 OH CH CH 2 C – CH 3 S – H CH 3 CH 2 C CH 3
B – or
CH CH 2 C C CH CH 3 Ph B – H Ph
3
2
+
Ph CH 3 O
C
CH 3 CH 2 CH 3
1
Retention of configuration was observed in nonpolar solvents, while increasing
amounts of inversion occurred as the proton-donating ability and the polarity of
the solvent increased. Cleavage of 1 with potassium t-butoxide in benzene gave
2-phenylbutane with 93% net retention of configuration. The stereochemical course
changed to 48% net inversion of configuration when potassium hydroxide in
ethylene glycol was used. In DMSO using K · O-t-Bu as base, completely racemic
+ −
2-phenylbutane was formed. 26 The retention in benzene presumably reflects a short
lifetime for the carbanion in a tight ion pair. Under these conditions, the carbanion
does not become symmetrically solvated before proton transfer from either the proto-
nated base or the ketone. The solvent benzene is not an effective proton donor and the
most likely proton source is t-butanol. In ethylene glycol, the solvent provides a good
proton source and since net inversion is observed, the protonation must occur on an
unsymmetrically solvated species that favors back-side protonation. The racemization
that is observed in DMSO indicates that the carbanion has a sufficient lifetime to
become symmetrically solvated. The stereochemistry observed in the three solvents is
in good accord with their solvating properties. In benzene, reaction occurs primarily
through ion pairs. Ethylene glycol provides a ready source of protons and fast proton
transfer accounts for the observed inversion. DMSO promotes ion pair dissociation
and equilibration, as indicated by the observed racemization.
The stereochemistry of hydrogen-deuterium exchange at the chiral carbon in
2-phenylbutane shows a similar trend. When potassium t-butoxide is used as the base,
the exchange occurs with retention of configuration in t-butanol, but racemization
occurs in DMSO. 27 The retention of configuration is visualized as occurring through
an ion pair in which a solvent molecule coordinated to the metal ion acts as the proton
donor. In DMSO, symmetrical solvation is achieved prior to protonation and there is
complete racemization.
R R R
CH 3 CH 2 D O R CH 3 CH 2 D O R CH 3 CH 2 – O R
CH 3 C H + K + O CH 3 C H K + O CH 3 C D H K + O
Ph O – D Ph O D Ph O D
R R R
exchange with retention
of configuration
26 D. J. Cram, A. Langemann, J. Allinger, and K. R. Kopecky, J. Am. Chem. Soc., 81, 5740 (1959).
27
D. J. Cram, C. A. Kingsbury, and B. Rickborn, J. Am. Chem. Soc., 83, 3688 (1961).