Page 608 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 608
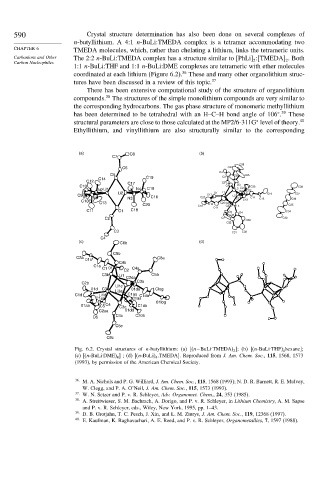
590 Crystal structure determination has also been done on several complexes of
n-butyllithium. A 4:1 n-BuLi:TMEDA complex is a tetramer accommodating two
CHAPTER 6 TMEDA molecules, which, rather than chelating a lithium, links the tetrameric units.
Carbanions and Other The 2:2 n-BuLi:TMEDA complex has a structure similar to [PhLi] :[TMEDA] . Both
Carbon Nucleophiles 2 2
1:1 n-BuLi:THF and 1:1 n-BuLi:DME complexes are tetrameric with ether molecules
36
coordinated at each lithium (Figure 6.2). These and many other organolithium struc-
tures have been discussed in a review of this topic. 37
There has been extensive computational study of the structure of organolithium
38
compounds. The structures of the simple monolithium compounds are very similar to
the corresponding hydrocarbons. The gas phase structure of monomeric methyllithium
39
has been determined to be tetrahedral with an H–C–H bond angle of 106 . These
structural parameters are close to those calculated at the MP2/6-311G level of theory. 40
∗
Ethyllithium, and vinyllithium are also structurally similar to the corresponding
(a) C8 (b)
C7
C26
C6 C27
C4
C5 C28 C25
C14 C19 C3
C12 C2
C10 C17 C1 D3 C23 C38
N2 Li1 N4 C15 L3 C24 C22
Li2 D1 L1 D2
C9 C16 C17 L2 C16 C37
N1 N3 C20 C21 C14 C15 C36
C10E C13 C13
C20 C19 C12 C20 C2 L4 C35
C3
C11
C11 C1 C18 C9 D4 C34
C8
C2 C7 C29 C33
C32
C3 C31 C30
C4
(c) C6b (d)
C5b
C2a C8a
01a
C4b
C1a
C1 01 C2 C4a
C3b Li1 C5a
C2da
C2b C3a
Li1c
01d C3 01da Clog
Li3a
C1d Li1d C1aa C1da
C1aa 01aa
01log
01aa C5 C4 C3c C1db
C2aa 01dd
C6 C4c C2db
C5c
C8c
Fig. 6.2. Crystal structures of n-butyllithium: (a) [(n−BuLi TMEDA) 2 ]; (b) [(n-BuLi THF) hexane];
4
(c) [(n-BuLi DME) 4 ] ; (d) [(n-BuLi) 4 .TMEDA]. Reproduced from J. Am. Chem. Soc., 115, 1568, 1573
(1993), by permission of the American Chemical Society.
36 M. A. Nichols and P. G. Williard, J. Am. Chem. Soc., 115, 1568 (1993); N. D. R. Barnett, R. E. Mulvey,
W. Clegg, and P. A. O’Neil, J. Am. Chem. Soc., 115, 1573 (1993).
37 W. N. Setzer and P. v. R. Schleyer, Adv. Organomet. Chem., 24, 353 (1985).
38
A. Streitwieser, S. M. Bachrach, A. Dorigo, and P. v. R. Schleyer, in Lithium Chemistry, A. M. Sapse
and P. v. R. Schleyer, eds., Wiley, New York, 1995, pp. 1–43.
39 D. B. Grotjahn, T. C. Pesch, J. Xin, and L. M. Ziurys, J. Am. Chem. Soc., 119, 12368 (1997).
40
E. Kaufman, K. Raghavachari, A. E. Reed, and P. v. R. Schleyer, Organometallics, 7, 1597 (1988).