Page 607 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 607
TheTHFsolvateoflithiumt-butylacetylideisanotherexampleofatetramericstructure. 31 589
In solutions of n-propyllithium in cyclopropane at 0 C, the hexamer is the main species,
but higher aggregates are present at lower temperatures. 20 SECTION 6.2
The reactivity of the organolithium compounds is increased by adding molecules Carbanion Character of
Organometallic
capable of solvating the lithium cations. Tetramethylenediamine (TMEDA) is commonly Compounds
used for organolithium reagents. This tertiary diamine can chelate lithium. The resulting
32
complexes generally are able to effect deprotonation at accelerated rates. In the case
of phenyllithium, NMR studies show that the compound is tetrameric in 1:2 ether-
cyclohexane, but dimeric in 1:9 TMEDA-cyclohexane. 33
(CH ) N N(CH )
3 2
R R 3 2
Li Li
Li Li + 4(CH ) NCH CH N(CH ) 2 R R
2
3 2
2
3 2
Li R Li
R (CH ) N N(CH )
3 2
3 2
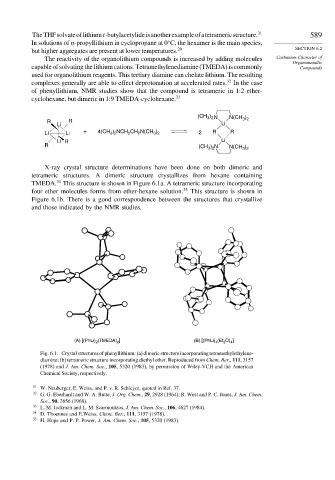
X-ray crystal structure determinations have been done on both dimeric and
tetrameric structures. A dimeric structure crystallizes from hexane containing
34
TMEDA. This structure is shown in Figure 6.1a. A tetrameric structure incorporating
four ether molecules forms from ether-hexane solution. 35 This structure is shown in
Figure 6.1b. There is a good correspondence between the structures that crystallize
and those indicated by the NMR studies.
(A) [(PhLi) 2 (TMEDA) 2 ] (B) [(PhLi) 4 (Et 2 O) 4 ]
Fig. 6.1. Crystal structures of phenyllithium: (a) dimeric structure incorporating tetramethylethylene-
diamine; (b) tetrameric structure incorporating diethyl ether. Reproduced from Chem. Ber., 111, 3157
(1978) and J. Am. Chem. Soc., 105, 5320 (1983), by permission of Wiley-VCH and the American
Chemical Society, respectively.
31 W. Neuberger, E. Weiss, and P. v. R. Schleyer, quoted in Ref. 37.
32
G. G. Eberhardt and W. A. Butte, J. Org. Chem., 29, 2928 (1964); R. West and P. C. Jones, J. Am. Chem.
Soc., 90, 2656 (1968).
33 L. M. Jackman and L. M. Scarmoutzos, J. Am. Chem. Soc., 106, 4627 (1984).
34 D. Thoennes and E.Weiss, Chem. Ber., 111, 3157 (1978).
35
H. Hope and P. P. Power, J. Am. Chem. Soc., 105, 5320 (1983).