Page 611 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 611
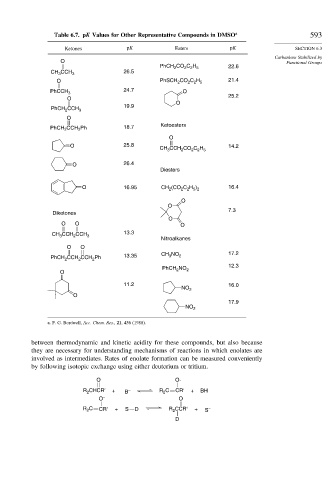
Table 6.7. pK Values for Other Representative Compounds in DMSO a 593
Ketones pK Esters pK SECTION 6.3
Carbanions Stabilized by
O Functional Groups
PhCH CO C H 22.6
2
2 2 5
CH CCH 3 26.5
3
O PhSCH CO C H 21.4
2 2 5
2
24.7
PhCCH 3 O
25.2
O
O
PhCH CCH 3 19.9
2
O
PhCH 2 CCH 2 Ph 18.7 Ketoesters
O
O 25.8 14.2
CH CCH CO C H
3
2 2 5
2
O 26.4
Diesters
O 16.95 CH (CO C H ) 16.4
2
2 2 5 2
O
O
7.3
Diketones
O
O O O
CH CCH CCH 3 13.3
3
2
Nitroalkanes
O O
2
2
PhCH 2 CCH CCH Ph 13.35 CH 3 NO 2 17.2
12.3
PhCH NO
O 2 2
11.2 16.0
NO 2
O
17.9
NO 2
a. F. G. Bordwell, Acc. Chem. Res., 21, 456 (1988).
between thermodynamic and kinetic acidity for these compounds, but also because
they are necessary for understanding mechanisms of reactions in which enolates are
involved as intermediates. Rates of enolate formation can be measured conveniently
by following isotopic exchange using either deuterium or tritium.
O O-
CHCR' – R C CR'
R 2 + B 2 + BH
O – O
R C CR' + S D R CCR' + S –
2
2
D