Page 616 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 616
598
3.08Å
CHAPTER 6
2.5Å
Carbanions and Other
Carbon Nucleophiles
3.8 kcal/mol 0.0 kcal/mol
2.26Å
2.45Å
2.45Å 2.34Å
0.0 kcal/mol 2.8 kcal/mol
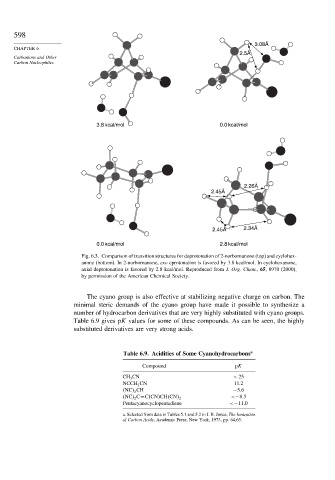
Fig. 6.3. Comparison of transition structures for deprotonation of 2-norbornanone (top) and cyclohex-
anone (bottom). In 2-norbornanone, exo eprotonation is favored by 3.8 kcal/mol. In cyclohexanone,
axial deprotonation is favored by 2.8 kcal/mol. Reproduced from J. Org. Chem., 65, 8970 (2000),
by permission of the American Chemical Society.
The cyano group is also effective at stabilizing negative charge on carbon. The
minimal steric demands of the cyano group have made it possible to synthesize a
number of hydrocarbon derivatives that are very highly substituted with cyano groups.
Table 6.9 gives pK values for some of these compounds. As can be seen, the highly
substituted derivatives are very strong acids.
Table 6.9. Acidities of Some Cyanohydrocarbons a
Compound pK
CH 3 CN > 25
NCCH 2 CN 11.2
NC 3 CH −5 6
<−8 5
NC 2 C=C CN CH CN 2
Pentacyanocyclopentadiene <−11 0
a. Selected from data in Tables 5.1 and 5.2 in J. R. Jones, The Ionization
of Carbon Acids, Academic Press, New York, 1973, pp. 64,65.