Page 620 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 620
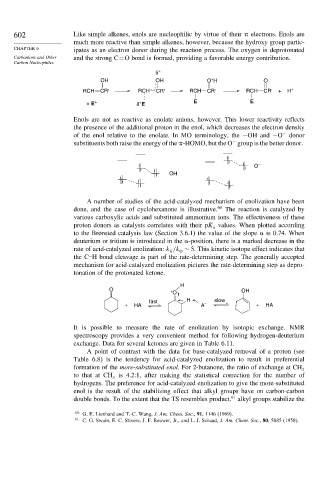
602 Like simple alkenes, enols are nucleophilic by virtue of their electrons. Enols are
much more reactive than simple alkenes, however, because the hydroxy group partic-
CHAPTER 6 ipates as an electron donor during the reaction process. The oxygen is deprotonated
Carbanions and Other and the strong C=O bond is formed, providing a favorable energy contribution.
Carbon Nucleophiles
δ +
+
OH OH O H O
+
RCH CR' RCH CR' RCH CR' RCH CR' + H
+
+ E + δ E E E
Enols are not as reactive as enolate anions, however. This lower reactivity reflects
the presence of the additional proton in the enol, which decreases the electron density
of the enol relative to the enolate. In MO terminology, the −OH and −O donor
−
substituents both raise the energy of the -HOMO, but the O group is the better donor.
−
O –
OH
A number of studies of the acid-catalyzed mechanism of enolization have been
done, and the case of cyclohexanone is illustrative. 60 The reaction is catalyzed by
various carboxylic acids and substituted ammonium ions. The effectiveness of these
proton donors as catalysts correlates with their pK values. When plotted according
a
to the Brønsted catalysis law (Section 3.6.1) the value of the slope is 0.74. When
deuterium or tritium is introduced in the -position, there is a marked decrease in the
rate of acid-catalyzed enolization: k /k ∼ 5. This kinetic isotope effect indicates that
H
D
the C–H bond cleavage is part of the rate-determining step. The generally accepted
mechanism for acid-catalyzed enolization pictures the rate-determining step as depro-
tonation of the protonated ketone.
H
O + OH
O
fast H slow
+ HA A – + HA
It is possible to measure the rate of enolization by isotopic exchange. NMR
spectroscopy provides a very convenient method for following hydrogen-deuterium
exchange. Data for several ketones are given in Table 6.11.
A point of contrast with the data for base-catalyzed removal of a proton (see
Table 6.8) is the tendency for acid-catalyzed enolization to result in preferential
formation of the more-substituted enol. For 2-butanone, the ratio of exchange at CH 2
to that at CH is 4.2:1, after making the statistical correction for the number of
3
hydrogens. The preference for acid-catalyzed enolization to give the more-substituted
enol is the result of the stabilizing effect that alkyl groups have on carbon-carbon
61
double bonds. To the extent that the TS resembles product, alkyl groups stabilize the
60 G. E. Lienhard and T.-C. Wang, J. Am. Chem. Soc., 91, 1146 (1969).
61
C. G. Swain, E. C. Stivers, J. F. Reuwer, Jr., and L. J. Schaad, J. Am. Chem. Soc., 80, 5885 (1958).