Page 621 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 621
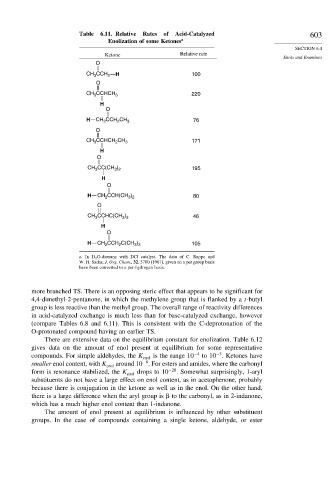
Table 6.11. Relative Rates of Acid-Catalyzed 603
Enolization of some Ketones a
SECTION 6.4
Ketone Relative rate
Enols and Enamines
O
CCH
CH 3 2 H 100
O
CH CCHCH 3 220
3
H
O
H CH CCH CH 3 76
2
2
O
CH CCHCH CH 3 171
3
2
H
O
CH CC(CH ) 195
3
3 2
H
O
H CH 2 CCH(CH ) 80
3 2
O
CH CCHC(CH ) 46
3 3
3
H
O
H CH 2 CCH C(CH ) 105
2
3 3
a. In D 2 O-dioxane with DCl catalyst. The data of C. Rappe and
W. H. Sachs, J. Org. Chem., 32, 3700 (1967), given on a per group basis
have been converted to a per-hydrogen basis.
more branched TS. There is an opposing steric effect that appears to be significant for
4,4-dimethyl-2-pentanone, in which the methylene group that is flanked by a t-butyl
group is less reactive than the methyl group. The overall range of reactivity differences
in acid-catalyzed exchange is much less than for base-catalyzed exchange, however
(compare Tables 6.8 and 6.11). This is consistent with the C-deprotonation of the
O-protonated compound having an earlier TS.
There are extensive data on the equilibrium constant for enolization. Table 6.12
gives data on the amount of enol present at equilibrium for some representative
−5
compounds. For simple aldehydes, the K enol is the range 10 −4 to 10 . Ketones have
−8
smaller enol content, with K enol around 10 . For esters and amides, where the carbonyl
form is resonance stabilized, the K enol drops to 10 −20 . Somewhat surprisingly, 1-aryl
substituents do not have a large effect on enol content, as in acetophenone, probably
because there is conjugation in the ketone as well as in the enol. On the other hand,
there is a large difference when the aryl group is
to the carbonyl, as in 2-indanone,
which has a much higher enol content than 1-indanone.
The amount of enol present at equilibrium is influenced by other substituent
groups. In the case of compounds containing a single ketone, aldehyde, or ester