Page 622 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 622
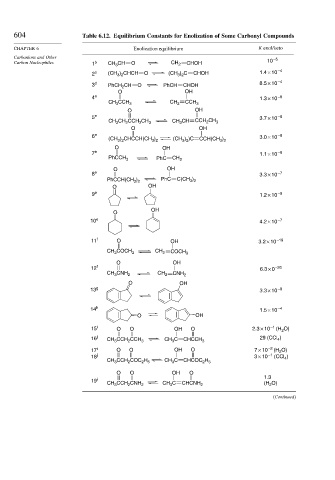
604 Table 6.12. Equilibrium Constants for Enolization of Some Carbonyl Compounds
CHAPTER 6 Enolization equilibrium K enol/keto
Carbanions and Other –5
Carbon Nucleophiles 1 b CH 3 CH O CH 2 CHOH 10
2 c (CH 3 ) 2 CHCH O (CH 3 ) 2 C CHOH 1.4 × 10 –4
–4
3 d PhCH 2 CH O PhCH CHOH 8.5 × 10
O OH
4 e 1.3 × 10 –8
CH 3 CCH 3 CH 2 CCH 3
O OH
5 e 3.7 × 10 –8
CH 3 CH 2 CCH 2 CH 3 CH 3 CH CCH 2 CH 3
O OH
6 e 3.0 × 10 –8
(CH 3 ) 2 CHCCH(CH 3 ) 2 (CH 3 ) 2 )C CCH(CH 3 ) 2
O OH
7 e 1.1 × 10 –8
PhC
PhCCH 3 CH 2
O OH
8 e 3.3 × 10 –7
PhC
PhCCH(CH 3 ) 2 C(CH 3 ) 2
O OH
9 e 1.2 × 10 –8
OH
O
10 e 4.2 × 10 –7
11 f O OH 3.2 × 10 –19
CH 3 COCH 3 CH 2 COCH 3
O OH
12 f 6.3 × 0 –20
CH 3 CNH 2 CH 2 CNH 2
O OH
13 g 3.3 × 10 –8
14 h –4
1.5 × 10
O OH
15 i O O OH O 2.3 × 10 –1 (H 2 O)
16 j CH 3 CCH 2 CCH 3 CH 3 C CHCCH 3 29 (CCl 4 )
17 i O O OH O 7 × 10 –2 (H 2 O)
–1
18 j 3 × 10 (CCl 4 )
CH 3 CCH 2 COC 2 H 5 CH 3 C CHCOC 2 H 5
O O OH O
1.3
19 i
CH 3 CCH 2 CNH 2 CH 3 C CHCNH 2 (H 2 O)
(Continued)