Page 624 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 624
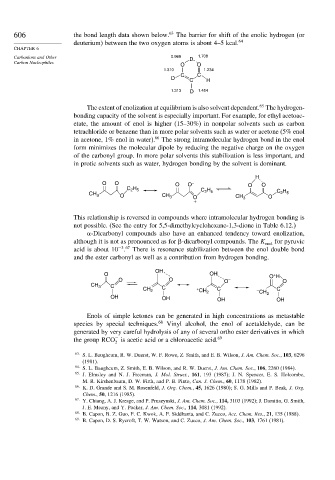
606 the bond length data shown below. 63 The barrier for shift of the enolic hydrogen (or
deuterium) between the two oxygen atoms is about 4–5 kcal. 64
CHAPTER 6
Carbanions and Other 0.969 D 1.708
Carbon Nucleophiles
O O
1.310 1.234
C C
D C H
1.313 D 1.454
65
The extent of enolization at equilibrium is also solvent dependent. The hydrogen-
bonding capacity of the solvent is especially important. For example, for ethyl acetoac-
etate, the amount of enol is higher (15–30%) in nonpolar solvents such as carbon
tetrachloride or benzene than in more polar solvents such as water or acetone (5% enol
in acetone, 1% enol in water). 66 The strong intramolecular hydrogen bond in the enol
form minimizes the molecular dipole by reducing the negative charge on the oxygen
of the carbonyl group. In more polar solvents this stabilization is less important, and
in protic solvents such as water, hydrogen bonding by the solvent is dominant.
H
O O O O – O O
C H H
H
2 5
CH 3 O CH 3 O C 2 5 CH O C 2 5
+ 3
This relationship is reversed in compounds where intramolecular hydrogen bonding is
not possible. (See the entry for 5,5-dimethylcyclohexane-1,3-dione in Table 6.12.)
-Dicarbonyl compounds also have an enhanced tendency toward enolization,
although it is not as pronounced as for
-dicarbonyl compounds. The K enol for pyruvic
−3 67
acid is about 10 . There is resonance stabilization between the enol double bond
and the ester carbonyl as well as a contribution from hydrogen bonding.
OH
O OH O H
+
O O O – O
CH 3 C
CH 2 C + CH 2 C – CH C
OH OH OH 2 OH
Enols of simple ketones can be generated in high concentrations as metastable
species by special techniques. 68 Vinyl alcohol, the enol of acetaldehyde, can be
generated by very careful hydrolysis of any of several ortho ester derivatives in which
−
the group RCO is acetic acid or a chloroacetic acid. 69
2
63
S. L. Baughcum, R. W. Duerst, W. F. Rowe, Z. Smith, and E. B. Wilson, J. Am. Chem. Soc., 103, 6296
(1981).
64 S. L. Baughcum, Z. Smith, E. B. Wilson, and R. W. Duerst, J. Am. Chem. Soc., 106, 2260 (1984).
65 J. Elmsley and N. J. Freeman, J. Mol. Struct., 161, 193 (1987); J. N. Spencer, E. S. Holcombe,
M. R. Kirshenbaum, D. W. Firth, and P. B. Pinto, Can. J. Chem., 60, 1178 (1982).
66
K. D. Grande and S. M. Rosenfeld, J. Org. Chem., 45, 1626 (1980); S. G. Mills and P. Beak, J. Org.
Chem., 50, 1216 (1985).
67 Y. Chiang, A. J. Kresge, and P. Pruszynski, J. Am. Chem. Soc., 114, 3103 (1992); J. Damitio, G. Smith,
J. E. Meany, and Y. Pocker, J. Am. Chem. Soc., 114, 3081 (1992).
68 B. Capon, B. Z. Guo, F. C. Kwok, A. F. Siddhanta, and C. Zucco, Acc. Chem. Res., 21, 135 (1988).
69
B. Capon, D. S. Rycroft, T. W. Watson, and C. Zucco, J. Am. Chem. Soc., 103, 1761 (1981).