Page 625 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 625
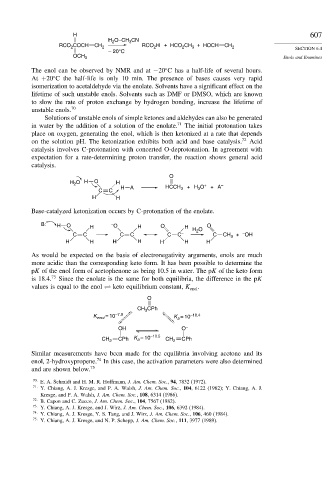
H 607
H O–CH CN
3
2
RCO COCH CH 2 RCO H + HCO CH 3 + HOCH CH 2 SECTION 6.4
2
2
2
– 20°C
OCH 3 Enols and Enamines
The enol can be observed by NMR and at −20 C has a half-life of several hours.
At +20 C the half-life is only 10 min. The presence of bases causes very rapid
isomerization to acetaldehyde via the enolate. Solvents have a significant effect on the
lifetime of such unstable enols. Solvents such as DMF or DMSO, which are known
to slow the rate of proton exchange by hydrogen bonding, increase the lifetime of
unstable enols. 70
Solutions of unstable enols of simple ketones and aldehydes can also be generated
in water by the addition of a solution of the enolate. 71 The initial protonation takes
place on oxygen, generating the enol, which is then ketonized at a rate that depends
on the solution pH. The ketonization exhibits both acid and base catalysis. 72 Acid
catalysis involves C-protonation with concerted O-deprotonation. In agreement with
expectation for a rate-determining proton transfer, the reaction shows general acid
catalysis.
O
O H O H
H 2 + –
H A HCCH + H O + A
C C 3 3
H H
Base-catalyzed ketonization occurs by C-protonation of the enolate.
B: – H O H – O H O H H O O
C C C C C C – 2 C CH 3 + – OH
H H H H H H H
As would be expected on the basis of electronegativity arguments, enols are much
more acidic than the corresponding keto form. It has been possible to determine the
pK of the enol form of acetophenone as being 10.5 in water. The pK of the keto form
is 18.4. 73 Since the enolate is the same for both equilibria, the difference in the pK
values is equal to the enol keto equilibrium constant, K enol .
O
CH CPh
3
K enol = 10 –7.9 K a = 10 –18.4
OH O –
CH 2 CPh K = 10 –10.5 CH 2 CPh
a
Similar measurements have been made for the equilibria involving acetone and its
74
enol, 2-hydroxypropene. In this case, the activation parameters were also determined
and are shown below. 75
70
E. A. Schmidt and H. M. R. Hoffmann, J. Am. Chem. Soc., 94, 7832 (1972).
71 Y. Chiang, A. J. Kresge, and P. A. Walsh, J. Am. Chem. Soc., 104, 6122 (1982); Y. Chiang, A. J.
Kresge, and P. A. Walsh, J. Am. Chem. Soc., 108, 6314 (1986).
72
B. Capon and C. Zucco, J. Am. Chem. Soc., 104, 7567 (1982).
73 Y. Chiang, A. J. Kresge, and J. Wirz, J. Am. Chem. Soc., 106, 6392 (1984).
74 Y. Chiang, A. J. Kresge, Y. S. Tang, and J. Wirz, J. Am. Chem. Soc., 106, 460 (1984).
75
Y. Chiang, A. J. Kresge, and N. P. Schepp, J. Am. Chem. Soc., 111, 3977 (1989).