Page 630 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 630
612 O – + Li
O – + Li
CHAPTER 6 CH 3
CH
Carbanions and Other 3 CH 3 4
Carbon Nucleophiles CH 3
Detailed investigation of the degree of aggregation in solution has been applied
to several alkyl aryl ketones. 82 The lithium enolate of 4-(4-biphenyl)-2-methyl-1-
propanone in THF exhibits a monomer-tetramer equilibrium. 83 The K eq for tetramer-
3
8
ization is estimated as 5 × 10 M , which corresponds to 1.3% of the enolate being
present as the monomer. The kinetics of the alkylation reaction with benzyl bromide
indicates that the monomer is the reactive nucleophile. Related studies were carried out
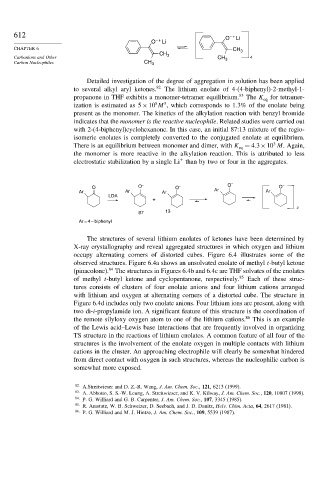
with 2-(4-biphenyl)cyclohexanone. In this case, an initial 87:13 mixture of the regio-
isomeric enolates is completely converted to the conjugated enolate at equilibrium.
3
There is an equilibrium between monomer and dimer, with K = 4 3×10 M. Again,
eq
the monomer is more reactive in the alkylation reaction. This is attributed to less
+
electrostatic stabilization by a single Li than by two or four in the aggregates.
O O – O – O – O –
Ar Ar Ar Ar Ar
LDA
+
2
87 13
Ar = 4 – biphenyl
The structures of several lithium enolates of ketones have been determined by
X-ray crystallography and reveal aggregated structures in which oxygen and lithium
occupy alternating corners of distorted cubes. Figure 6.4 illustrates some of the
observed structures. Figure 6.4a shows an unsolvated enolate of methyl t-butyl ketone
84
(pinacolone). The structures in Figures 6.4b and 6.4c are THF solvates of the enolates
of methyl t-butyl ketone and cyclopentanone, respectively. 85 Each of these struc-
tures consists of clusters of four enolate anions and four lithium cations arranged
with lithium and oxygen at alternating corners of a distorted cube. The structure in
Figure 6.4d includes only two enolate anions. Four lithium ions are present, along with
two di-i-propylamide ion. A significant feature of this structure is the coordination of
the remote silyloxy oxygen atom to one of the lithium cations. 86 This is an example
of the Lewis acid–Lewis base interactions that are frequently involved in organizing
TS structure in the reactions of lithium enolates. A common feature of all four of the
structures is the involvement of the enolate oxygen in multiple contacts with lithium
cations in the cluster. An approaching electrophile will clearly be somewhat hindered
from direct contact with oxygen in such structures, whereas the nucleophilic carbon is
somewhat more exposed.
82 A.Streitwieser and D. Z.-R. Wang, J. Am. Chem. Soc., 121, 6213 (1999).
83
A. Abbotto, S. S.-W. Leung, A. Streitwieser, and K. V. Kilway, J. Am. Chem. Soc., 120, 10807 (1998).
84 P. G. Williard and G. B. Carpenter, J. Am. Chem. Soc., 107, 3345 (1985).
85 R. Amstutz, W. B. Schweizer, D. Seebach, and J. D. Dunitz, Helv. Chim. Acta, 64, 2617 (1981).
86
P. G. Williard and M. J. Hintze, J. Am. Chem. Soc., 109, 5539 (1987).