Page 631 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 631
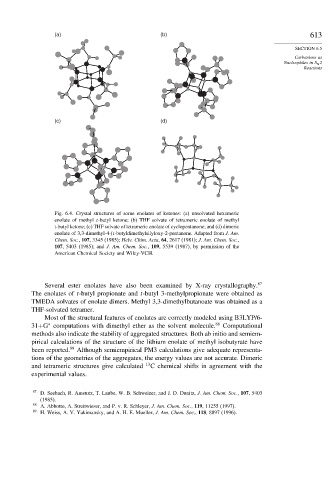
(a) (b) 613
SECTION 6.5
Carbanions as
Nucleophiles in S N 2
Reactions
(c) (d)
Fig. 6.4. Crystal structures of some enolates of ketones: (a) unsolvated hexameric
enolate of methyl t-butyl ketone; (b) THF solvate of tetrameric enolate of methyl
t-butyl ketone; (c) THF solvate of tetrameric enolate of cyclopentanone; and (d) dimeric
enolate of 3,3-dimethyl-4-(t-butyldimethylsilyloxy-2-pentanone. Adapted from J. Am.
Chem. Soc., 107, 3345 (1985); Helv. Chim. Acta, 64, 2617 (1981); J. Am. Chem. Soc.,
107, 5403 (1985); and J. Am. Chem. Soc., 109, 5539 (1987), by permission of the
American Chemical Society and Wiley-VCH.
Several ester enolates have also been examined by X-ray crystallography. 87
The enolates of t-butyl propionate and t-butyl 3-methylpropionate were obtained as
TMEDA solvates of enolate dimers. Methyl 3,3-dimethylbutanoate was obtained as a
THF-solvated tetramer.
Most of the structural features of enolates are correctly modeled using B3LYP/6-
31+G computations with dimethyl ether as the solvent molecule. 88 Computational
∗
methods also indicate the stability of aggregated structures. Both ab initio and semiem-
pirical calculations of the structure of the lithium enolate of methyl isobutyrate have
been reported. 89 Although semiempirical PM3 calculations give adequate representa-
tions of the geometries of the aggregates, the energy values are not accurate. Dimeric
and tetrameric structures give calculated 13 C chemical shifts in agreement with the
experimental values.
87
D. Seebach, R. Amstutz, T. Laube, W. B. Schweizer, and J. D. Dunitz, J. Am. Chem. Soc., 107, 5403
(1985).
88 A. Abbotto, A. Streitwieser, and P. v. R. Schleyer, J. Am. Chem. Soc., 119, 11255 (1997).
89
H. Weiss, A. V. Yakimansky, and A. H. E. Mueller, J. Am. Chem. Soc., 118, 8897 (1996).