Page 636 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 636
618
CHAPTER 6
Carbanions and Other
Carbon Nucleophiles
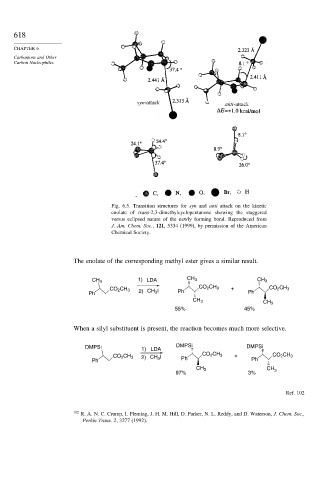
Fig. 6.5. Transition structures for syn and anti attack on the kinetic
enolate of trans-2,3-dimethylcyclopentanone showing the staggered
versus eclipsed nature of the newly forming bond. Reproduced from
J. Am. Chem. Soc., 121, 5334 (1999), by permission of the American
Chemical Society.
The enolate of the corresponding methyl ester gives a similar result.
1) LDA CH 3
CH 3 CH 3
CO 2 CH 3 + CO 2 CH 3
CO 2 CH 3 2) CH 3 I Ph
Ph Ph
CH 3
CH 3
55% 45%
When a silyl substituent is present, the reaction becomes much more selective.
DMPSi 1) LDA DMPSi DMPSi
CO 2 CH 3 2) CH 3 I Ph CO 2 CH 3 + CO 2 CH 3
Ph Ph
CH 3 CH 3
97% 3%
Ref. 102
102
R. A. N. C. Crump, I. Fleming, J. H. M. Hill, D. Parker, N. L. Reddy, and D. Waterson, J. Chem. Soc.,
Perkin Trans. 2, 3277 (1992).