Page 638 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 638
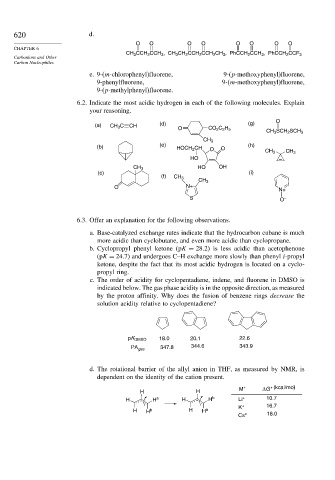
620 d.
O O O O O O O O
CHAPTER 6
CH 3 CCH 2 CCH 3 , CH 3 CH 2 CCH 2 CCH 2 CH 3 , PhCCH 2 CCH 3 , PhCCH 2 CCF 3
Carbanions and Other
Carbon Nucleophiles
e. 9-(m-chlorophenyl)fluorene, 9-(p-methoxyphenyl)fluorene,
9-phenylfluorene, 9-(m-methoxyphenyl)fluorene,
9-(p-methylphenyl)fluorene.
6.2. Indicate the most acidic hydrogen in each of the following molecules. Explain
your reasoning.
O
(a) CH 3 C CH (d) (g)
O CO 2 C 2 H 5
CH 3 SCH 2 SCH 3
CH 3
(b) (e) HOCH 2 CH O O (h)
CH 3 CH 3
HO
CH 3 HO OH
(c) (i)
(f) CH 3
CH 3
O N+ N+
S O –
6.3. Offer an explanation for the following observations.
a. Base-catalyzed exchange rates indicate that the hydrocarbon cubane is much
more acidic than cyclobutane, and even more acidic than cyclopropane.
b. Cyclopropyl phenyl ketone (pK = 28 2) is less acidic than acetophenone
(pK = 24 7) and undergoes C–H exchange more slowly than phenyl i-propyl
ketone, despite the fact that its most acidic hydrogen is located on a cyclo-
propyl ring.
c. The order of acidity for cyclopentadiene, indene, and fluorene in DMSO is
indicated below. The gas phase acidity is in the opposite direction, as measured
by the proton affinity. Why does the fusion of benzene rings decrease the
solution acidity relative to cyclopentadiene?
pK DMSO 18.0 20.1 22.6
PA gas 347.8 344.6 343.9
d. The rotational barrier of the allyl anion in THF, as measured by NMR, is
dependent on the identity of the cation present.
M + ΔG* (kcal/mol)
H H
H H a H H b Li + 10.7
K + 16.7
H H b H H a
Cs + 18.0