Page 633 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 633
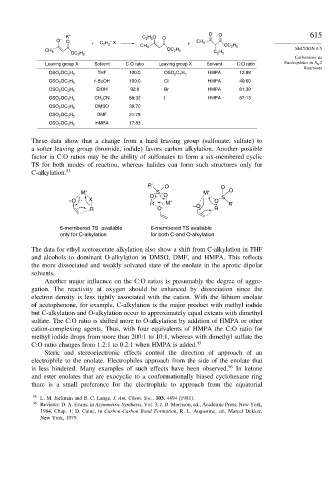
K + C 2 H 5 O O O O 615
O – O + C 2 H 5 X + CH 3
–
CH 3 OC 2 H 5
SECTION 6.5
OC 2 H 5
CH 3 C 2 H 5
OC 2 H 5
Carbanions as
Leaving group X Solvent C:O ratio Leaving group X Solvent C:O ratio Nucleophiles in S N 2
Reactions
THF 100:0 HMPA 12:88
OSO 2 OC 2 H 5 OSO 2 C 7 H 7
t -BuOH 100:0 Cl HMPA 40:60
OSO 2 OC 2 H 5
EtOH 92:8 Br HMPA 61:39
OSO 2 OC 2 H 5
CH 3 CN 68:32 I HMPA 87:13
OSO 2 OC 2 H 5
DMSO 30:70
OSO 2 OC 2 H 5
DMF 21:79
OSO 2 OC 2 H 5
HMPA 17:83
OSO 2 OC 2 H 5
These data show that a change from a hard leaving group (sulfonate, sulfate) to
a softer leaving group (bromide, iodide) favors carbon alkylation. Another possible
factor in C:O ratios may be the ability of sulfonates to form a six-membered cyclic
TS for both modes of reaction, whereas halides can form such structures only for
C-alkylation. 83
R' O O
M + S M + O
O O S
–O X + O
R M –O R'
R O - R
6-membered TS available 6-membered TS available
only for C-alkylation for both C-and O-alkylation
The data for ethyl acetoacetate alkylation also show a shift from C-alkylation in THF
and alcohols to dominant O-alkylation in DMSO, DMF, and HMPA. This reflects
the more dissociated and weakly solvated state of the enolate in the aprotic dipolar
solvents.
Another major influence on the C:O ratios is presumably the degree of aggre-
gation. The reactivity at oxygen should be enhanced by dissociation since the
electron density is less tightly associated with the cation. With the lithium enolate
of acetophenone, for example, C-alkylation is the major product with methyl iodide
but C-alkylation and O-alkylation occur to approximately equal extents with dimethyl
sulfate. The C:O ratio is shifted more to O-alkylation by addition of HMPA or other
cation-complexing agents. Thus, with four equivalents of HMPA the C:O ratio for
methyl iodide drops from more than 200:1 to 10:1, whereas with dimethyl sulfate the
C:O ratio changes from 1.2:1 to 0.2:1 when HMPA is added. 95
Steric and stereoelectronic effects control the direction of approach of an
electrophile to the enolate. Electrophiles approach from the side of the enolate that
is less hindered. Many examples of such effects have been observed. 96 In ketone
and ester enolates that are exocyclic to a conformationally biased cyclohexane ring
there is a small preference for the electrophile to approach from the equatorial
95 L. M. Jackman and B. C. Lange, J. Am. Chem. Soc., 103, 4494 (1981).
96
Reviews: D. A. Evans, in Asymmetric Synthesis, Vol. 3, J. D. Morrison, ed., Academic Press, New York,
1984, Chap. 1; D. Caine, in Carbon-Carbon Bond Formation, R. L. Augustine, ed., Marcel Dekker,
New York, 1979.