Page 629 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 629
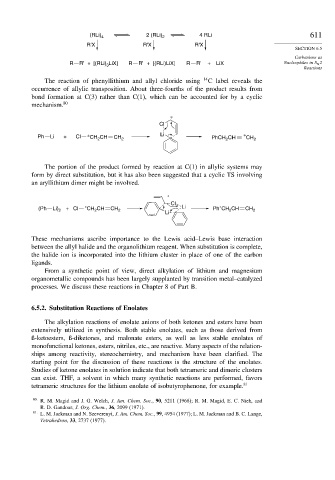
(RLi) 4 2 (RLi) 2 4 RLi 611
R'X R'X R'X
SECTION 6.5
Carbanions as
R R' + [(RLi) 3 LiX] R R' + [(RLi)LiX] R R' + LiX Nucleophiles in S N 2
Reactions
The reaction of phenyllithium and allyl chloride using 14 C label reveals the
occurrence of allylic transposition. About three-fourths of the product results from
bond formation at C(3) rather than C(1), which can be accounted for by a cyclic
mechanism. 80
∗
Cl
Li ∗
Ph Li + Cl ∗ CH 2 CH CH 2 PhCH CH CH 2
2
The portion of the product formed by reaction at C(1) in allylic systems may
form by direct substitution, but it has also been suggested that a cyclic TS involving
an aryllithium dimer might be involved.
∗
Cl
∗ Li ∗
(Ph Li) 2 + Cl CH CH CH 2 Ph CH CH CH 2
2
2
Li
These mechanisms ascribe importance to the Lewis acid–Lewis base interaction
between the allyl halide and the organolithium reagent. When substitution is complete,
the halide ion is incorporated into the lithium cluster in place of one of the carbon
ligands.
From a synthetic point of view, direct alkylation of lithium and magnesium
organometallic compounds has been largely supplanted by transition metal–catalyzed
processes. We discuss these reactions in Chapter 8 of Part B.
6.5.2. Substitution Reactions of Enolates
The alkylation reactions of enolate anions of both ketones and esters have been
extensively utilized in synthesis. Both stable enolates, such as those derived from
ß-ketoesters, ß-diketones, and malonate esters, as well as less stable enolates of
monofunctional ketones, esters, nitriles, etc., are reactive. Many aspects of the relation-
ships among reactivity, stereochemistry, and mechanism have been clarified. The
starting point for the discussion of these reactions is the structure of the enolates.
Studies of ketone enolates in solution indicate that both tetrameric and dimeric clusters
can exist. THF, a solvent in which many synthetic reactions are performed, favors
tetrameric structures for the lithium enolate of isobutyrophenone, for example. 81
80 R. M. Magid and J. G. Welch, J. Am. Chem. Soc., 90, 5211 (1968); R. M. Magid, E. C. Nieh, and
R. D. Gandour, J. Org. Chem., 36, 2099 (1971).
81
L. M. Jackman and N. Szeverenyi, J. Am. Chem. Soc., 99, 4954 (1977); L. M. Jackman and B. C. Lange,
Tetrahedron, 33, 2737 (1977).