Page 634 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 634
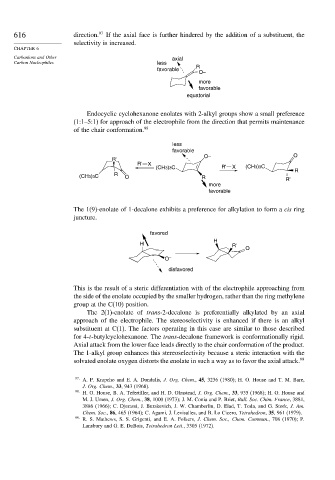
616 direction. 97 If the axial face is further hindered by the addition of a substituent, the
selectivity is increased.
CHAPTER 6
Carbanions and Other axial
Carbon Nucleophiles less
favorable R
O–
more
favorable
equatorial
Endocyclic cyclohexanone enolates with 2-alkyl groups show a small preference
(1:1–5:1) for approach of the electrophile from the direction that permits maintenance
of the chair conformation. 98
less
favorable
O– O
R'
R' X
(CH3)3C R' X (CH3)3C R
(CH3)3C R O R R'
more
favorable
The 1(9)-enolate of 1-decalone exhibits a preference for alkylation to form a cis ring
juncture.
favored
H
H R'
O
O –
disfavored
This is the result of a steric differentiation with of the electrophile approaching from
the side of the enolate occupied by the smaller hydrogen, rather than the ring methylene
group at the C(10) position.
The 2(1)-enolate of trans-2-decalone is preferentially alkylated by an axial
approach of the electrophile. The stereoselectivity is enhanced if there is an alkyl
substituent at C(1). The factors operating in this case are similar to those described
for 4-t-butylcyclohexanone. The trans-decalone framework is conformationally rigid.
Axial attack from the lower face leads directly to the chair conformation of the product.
The 1-alkyl group enhances this stereoselectivity because a steric interaction with the
solvated enolate oxygen distorts the enolate in such a way as to favor the axial attack. 99
97
A. P. Krapcho and E. A. Dundulis, J. Org. Chem., 45, 3236 (1980); H. O. House and T. M. Bare,
J. Org. Chem., 33, 943 (1968).
98 H. O. House, B. A. Tefertiller, and H. D. Olmstead, J. Org. Chem., 33, 935 (1968); H. O. House and
M. J. Umen, J. Org. Chem., 38, 1000 (1973); J. M. Conia and P. Briet, Bull. Soc. Chim. France, 3881,
3886 (1966); C. Djerassi, J. Burakevich, J. W. Chamberlin, D. Elad, T. Toda, and G. Stork, J. Am.
Chem. Soc., 86, 465 (1964); C. Agami, J. Levisalles, and B. Lo Cicero, Tetrahedron, 35, 961 (1979).
99
R. S. Mathews, S. S. Grigenti, and E. A. Folkers, J. Chem. Soc., Chem. Commun., 708 (1970); P.
Lansbury and G. E. DuBois, Tetrahedron Lett., 3305 (1972).