Page 635 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 635
H 617
H R
R R' X O
O – SECTION 6.5
Carbanions as
favored H R'
H Nucleophiles in S N 2
Reactions
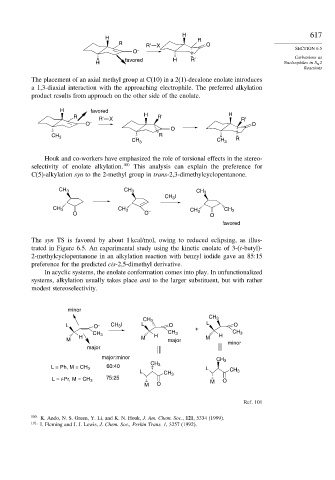
The placement of an axial methyl group at C(10) in a 2(1)-decalone enolate introduces
a 1,3-diaxial interaction with the approaching electrophile. The preferred alkylation
product results from approach on the other side of the enolate.
H favored
R R' X H R' H R'
O – O
O
R
CH 3
CH 3 CH 3 R
Houk and co-workers have emphasized the role of torsional effects in the stereo-
selectivity of enolate alkylation. 100 This analysis can explain the preference for
C(5)-alkylation syn to the 2-methyl group in trans-2,3-dimethylcyclopentanone.
CH 3 CH 3
CH 3
CH 3 I
CH 3 CH 3 – CH 3 CH 3
O O O
favored
The syn TS is favored by about 1 kcal/mol, owing to reduced eclipsing, as illus-
trated in Figure 6.5. An experimental study using the kinetic enolate of 3-(t-butyl)-
2-methylcyclopentanone in an alkylation reaction with benzyl iodide gave an 85:15
preference for the predicted cis-2,5-dimethyl derivative.
In acyclic systems, the enolate conformation comes into play. In unfunctionalized
systems, alkylation usually takes place anti to the larger substituent, but with rather
modest stereoselectivity.
minor
CH 3
CH 3 L
L O – CH 3 I L O O
+
CH 3 CH 3
CH 3 H H
H M M
M major
minor
major
major:minor
CH 3
60:40 CH 3
L = Ph, M = CH 3 L
L CH 3
CH 3
75:25
L = i-Pr, M = CH 3 M O
M O
Ref. 101
100 K. Ando, N. S. Green, Y. Li, and K. N. Houk, J. Am. Chem. Soc., 121, 5334 (1999).
101
I. Fleming and J. J. Lewis, J. Chem. Soc., Perkin Trans. 1, 3257 (1992).