Page 640 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 640
622
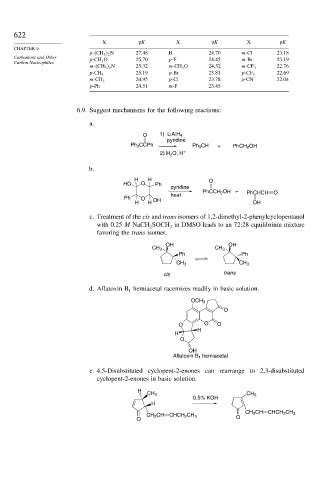
X pK X pK X pK
CHAPTER 6
p- CH 3 2 N 27.48 H 24.70 m-Cl 23.18
Carbanions and Other p-CH 3 O 25.70 p-F 24.45 m-Br 23.19
Carbon Nucleophiles
m- CH 3 2 N 25.32 m-CH 3 O 24.52 m-CF 3 22.76
25.19 p-Br 23.81 22.69
p-CH 3 p-CF 3
24.95 p-Cl 23.78 p-CN 22.04
m-CH 3
p-Ph 24.51 m-F 23.45
6.9. Suggest mechanisms for the following reactions:
a.
O 1) LiAlH 4
pyridine
Ph 3 CCPh Ph 3 CH + PhCH 2 OH
2) H O, H +
2
b.
H H O
HO O Ph
pyridine
PhCCH 2 OH + PhCHCH O
heat
Ph O
H H OH OH
c. Treatment of the cis and trans isomers of 1,2-dimethyl-2-phenylcyclopentanol
with 0.25 M NaCH SOCH in DMSO leads to an 72:28 equilibrium mixture
3
2
favoring the trans isomer.
OH OH
CH 3 CH 3
Ph Ph
CH 3 CH 3
cis trans
d. Aflatoxin B hemiacetal racemizes readily in basic solution.
1
OCH 3
O
O O O
H
H
O
OH
Aflatoxin B 1 hemiacetal
e. 4,5-Disubstituted cyclopent-2-enones can rearrange to 2,3-disubstituted
cyclopent-2-enones in basic solution.
H
CH 3 CH 3
0.5% KOH
H
CH 2 CH CHCH
CH 2 CH CHCH 2 CH 3 2 CH 3
O O