Page 645 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 645
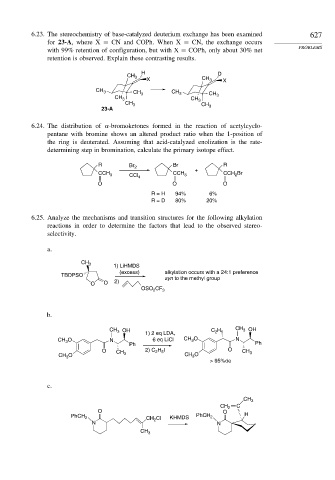
6.23. The stereochemistry of base-catalyzed deuterium exchange has been examined 627
for 23-A, where X = CN and COPh. When X = CN, the exchange occurs
PROBLEMS
with 99% retention of configuration, but with X = COPh, only about 30% net
retention is observed. Explain these contrasting results.
H D
CH 3
X CH 3 X
CH 3 CH CH 3 CH
CH 3 3 CH 3 3
CH 3 CH
23-A 3
6.24. The distribution of -bromoketones formed in the reaction of acetylcyclo-
pentane with bromine shows an altered product ratio when the 1-position of
the ring is deuterated. Assuming that acid-catalyzed enolization is the rate-
determining step in bromination, calculate the primary isotope effect.
R Br 2 Br R
CCH 3 CCl 4 CCH 3 + CCH Br
2
O O O
R = H 94% 6%
R = D 80% 20%
6.25. Analyze the mechanisms and transition structures for the following alkylation
reactions in order to determine the factors that lead to the observed stereo-
selectivity.
a.
CH 3
1) LiHMDS
(excess) alkylation occurs with a 24:1 preference
TBDPSO syn to the methyl group
O O 2)
CF
OSO 2 3
b.
CH OH CH 3 OH
3
1) 2 eq LDA, C 2 H 5
CH O N 6 eq LiCl CH O N
3
3
Ph Ph
O CH 3 2) C H I O CH 3
2 5
CH O CH O
3
3
> 95%de
c.
CH 3
CH 2 C
O O
PhCH 2 CH Cl KHMDS PhCH 2 H
2
N N
CH 3