Page 644 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 644
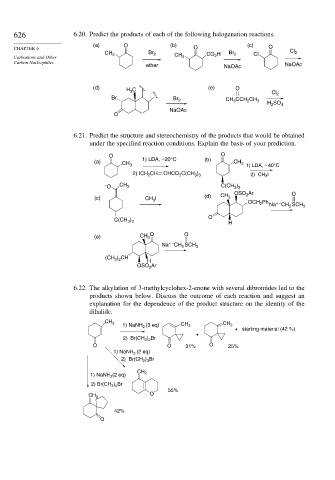
626 6.20. Predict the products of each of the following halogenation reactions.
(a) O (b) (c)
CHAPTER 6 O O
CH Br 2 CO H Br 2 Cl Cl 2
Carbanions and Other 3 CH 3 2
Carbon Nucleophiles
ether NaOAc NaOAc
(d) H C (e) O
3
Cl 2
Br Br 2 CH CCH CH
3
3
2
H SO 4
2
NaOAc
O
6.21. Predict the structure and stereochemistry of the products that would be obtained
under the specified reaction conditions. Explain the basis of your prediction.
O O
(a) 1) LDA, –20°C (b) CH 3
CH 3
1) LDA, – 40°C
2) ICH CH CHCO C(CH ) 2) CH 3 I
2
3 3
2
– O CH 3 C(CH )
3 3
Ar O
OSO 2
(c) CH I (d) CH 3
3
OCH 2 Ph + –
Na CH SCH 3
2
O
C(CH ) H
3 3
O O
(e) CH 3
+ –
Na CH SCH 3
2
) CH
(CH 3 2
H
OSO Ar
2
6.22. The alkylation of 3-methylcyclohex-2-enone with several dibromides led to the
products shown below. Discuss the outcome of each reaction and suggest an
explanation for the dependence of the product structure on the identity of the
dihalide.
CH 3
1) NaNH 2 (3 eq) CH 3 CH 2
+ starting material (42 %)
+
2) Br(CH 2 ) 2 Br
O O 31% O 25%
1) NaNH 2 (2 eq)
2) Br(CH 2 ) 3 Br
1) NaNH 2 (2 eq) CH 2
2) Br(CH 2 ) 4 Br
55%
O
CH 2
42%
O