Page 649 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 649
The nucleophile is shown as an anion, but can also be a neutral species, in which case 631
a proton is subsequently lost.
Introduction
There are carbonyl addition reactions that are examples of each of the general
mechanisms, and a two-dimensional potential energy diagram provides a useful
framework within which to consider specific addition reactions. The breakdown of
a tetrahedral intermediate involves the same processes but operates in the opposite
direction, so the principles that are developed also apply to the reactions of the tetra-
hedral intermediates. Let us examine the three general mechanistic cases in relation to
the energy diagram in Figure 7.1.
Case (a) is favored for weak nucleophiles. The protonated carbonyl compound is
more reactive toward such nucleophiles. The nucleophile may be neutral or a weakly
basic anion. This mechanism is most likely to operate in relatively acidic condi-
tions. Case (b) is favored for strongly basic nucleophiles. For example, carbanions
cannot generally exist under acidic conditions, so carbanion additions occur under
strongly basic conditions. These nucleophiles are more basic than carbonyl oxygens
and are protonated in preference to the carbonyl group. In such systems, proton
donors diminish the overall reaction rate by decreasing the amount of anionic nucle-
ophile that is available for reaction. The concerted mechanism, case (c), is observed
for less basic nucleophiles. The simultaneous transfer of the proton at the carbonyl
oxygen facilitates addition by species that are not sufficiently nucleophilic to react by
mechanism (b). The general pattern is that the weaker and less basic the nucleophile,
the more important the partial or complete protonation of the carbonyl group. If we
consider the reverse process, the same general relationships will hold. Good leaving
groups (which are poor nucleophiles) can be expected to follow path (a); poor leaving
groups will follow path (b); and intermediate cases are likely to react by the concerted
mechanism (c).
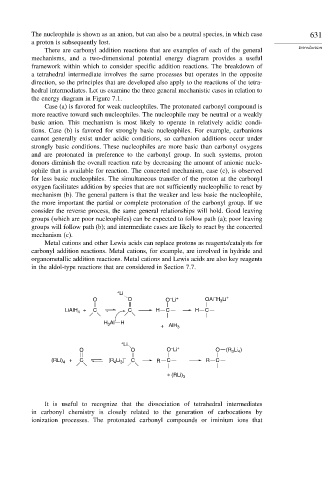
Metal cations and other Lewis acids can replace protons as reagents/catalysts for
carbonyl addition reactions. Metal cations, for example, are involved in hydride and
organometallic addition reactions. Metal cations and Lewis acids are also key reagents
in the aldol-type reactions that are considered in Section 7.7.
+ Li
–
–
O O O Li + OAl H Li +
3
LiAlH 4 + C C H C H C
H Al H
3
+ AlH 3
+ Li
–
O O O Li + O (R 3 Li )
4
(RLi) 4 + C [R Li ] – C R C R C
4
3
+ (RLi) 3
It is useful to recognize that the dissociation of tetrahedral intermediates
in carbonyl chemistry is closely related to the generation of carbocations by
ionization processes. The protonated carbonyl compounds or iminium ions that