Page 652 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 652
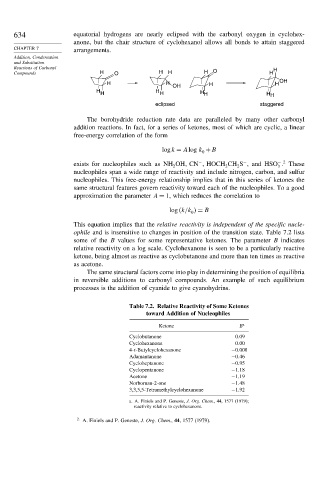
634 equatorial hydrogens are nearly eclipsed with the carbonyl oxygen in cyclohex-
anone, but the chair structure of cyclohexanol allows all bonds to attain staggered
CHAPTER 7 arrangements.
Addition, Condensation
and Substitution
Reactions of Carbonyl H
Compounds H O H H H O H
OH
H H H H
OH
H H H
H H H H H
eclipsed staggered
The borohydride reduction rate data are paralleled by many other carbonyl
addition reactions. In fact, for a series of ketones, most of which are cyclic, a linear
free-energy correlation of the form
logk = Alog k +B
0
− 2
−
−
exists for nucleophiles such as NH OH CN HOCH CH S , and HSO . These
2
3
2
2
nucleophiles span a wide range of reactivity and include nitrogen, carbon, and sulfur
nucleophiles. This free-energy relationship implies that in this series of ketones the
same structural features govern reactivity toward each of the nucleophiles. To a good
approximation the parameter A = 1, which reduces the correlation to
log k/k = B
0
This equation implies that the relative reactivity is independent of the specific nucle-
ophile and is insensitive to changes in position of the transition state. Table 7.2 lists
some of the B values for some representative ketones. The parameter B indicates
relative reactivity on a log scale. Cyclohexanone is seen to be a particularly reactive
ketone, being almost as reactive as cyclobutanone and more than ten times as reactive
as acetone.
The same structural factors come into play in determining the position of equilibria
in reversible additions to carbonyl compounds. An example of such equilibrium
processes is the addition of cyanide to give cyanohydrins.
Table 7.2. Relative Reactivity of Some Ketones
toward Addition of Nucleophiles
Ketone B a
Cyclobutanone 0 09
Cyclohexanone 0 00
4-t-Butylcyclohexanone −0 008
Adamantanone −0 46
Cycloheptanone −0 95
Cyclopentanone −1 18
Acetone −1 19
Norbornan-2-one −1 48
3,3,5,5-Tetramethylcyclohexanone −1 92
a. A. Finiels and P. Geneste, J. Org. Chem., 44, 1577 (1979);
reactivity relative to cyclohexanone.
2
A. Finiels and P. Geneste, J. Org. Chem., 44, 1577 (1979).