Page 655 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 655
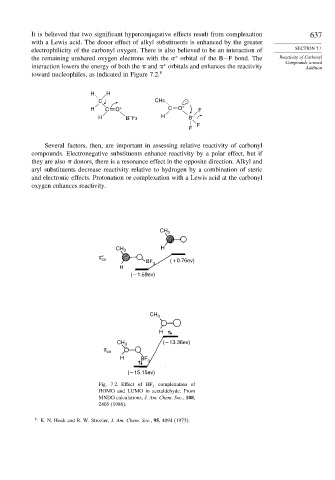
It is believed that two significant hyperconjugative effects result from complexation 637
with a Lewis acid. The donor effect of alkyl substituents is enhanced by the greater
electrophilicity of the carbonyl oxygen. There is also believed to be an interaction of SECTION 7.1
∗
the remaining unshared oxygen electrons with the orbital of the B−F bond. The Reactivity of Carbonyl
Compounds toward
∗
interaction lowers the energy of both the and orbitals and enhances the reactivity Addition
toward nucleophiles, as indicated in Figure 7.2. 8
H H
C CH3 :
H C O + C O + F
H B F3 H B –
–
F
F
Several factors, then, are important in assessing relative reactivity of carbonyl
compounds. Electronegative substituents enhance reactivity by a polar effect, but if
they are also donors, there is a resonance effect in the opposite direction. Alkyl and
aryl substituents decrease reactivity relative to hydrogen by a combination of steric
and electronic effects. Protonation or complexation with a Lewis acid at the carbonyl
oxygen enhances reactivity.
CH 3
CH 3 H
π ∗
co
BF ( + 0.76ev)
3
H
( – 1.59ev)
CH 3
H
CH 3 ( – 13.36ev)
π co
H BF
3
( – 15.15ev)
Fig. 7.2. Effect of BF 3 complexation of
HOMO and LUMO in acetaldehyde. From
MNDO calculations, J. Am. Chem. Soc., 108,
2405 (1986).
8
K. N. Houk and R. W. Strozier, J. Am. Chem. Soc., 95, 4094 (1973).