Page 660 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 660
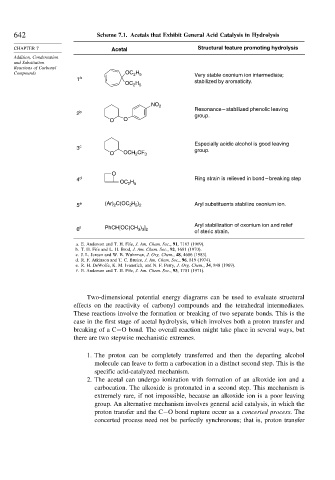
642 Scheme 7.1. Acetals that Exhibit General Acid Catalysis in Hydrolysis
CHAPTER 7 Acetal Structural feature promoting hydrolysis
Addition, Condensation
and Substitution
Reactions of Carbonyl
Compounds OC H Very stable oxonium ion intermediate;
2 5
1 a
OC H stabilized by aromaticity.
2 5
NO 2
Resonance – stabilized phenolic leaving
2 b group.
O O
Especially acidic alcohol is good leaving
3 c group.
O OCH 2 CF 3
O
4 d Ring strain is relieved in bond – breaking step
OC H
2 5
2
2 5 2
5 e (Ar) C(OC H ) Aryl substituents stabilize oxonium ion.
Aryl stabilization of oxonium ion and relief
) ]
6 f PhCH[OC(CH 3 3 2 of steric strain.
a. E. Anderson and T. H. Fife, J. Am. Chem. Soc., 91, 7163 (1969).
b. T. H. Fife and L. H. Brod, J. Am. Chem. Soc., 92, 1681 (1970).
c. J. L. Jensen and W. B. Wuhrman, J. Org. Chem., 48, 4686 (1983).
d. R. F. Atkinson and T. C. Bruice, J. Am. Chem. Soc., 96, 819 (1974).
e. R. H. DeWolfe, K. M. Ivanetich, and N. F. Perry, J. Org. Chem., 34, 848 (1969).
f. E. Anderson and T. H. Fife, J. Am. Chem. Soc., 93, 1701 (1971).
Two-dimensional potential energy diagrams can be used to evaluate structural
effects on the reactivity of carbonyl compounds and the tetrahedral intermediates.
These reactions involve the formation or breaking of two separate bonds. This is the
case in the first stage of acetal hydrolysis, which involves both a proton transfer and
breaking of a C−O bond. The overall reaction might take place in several ways, but
there are two stepwise mechanistic extremes.
1. The proton can be completely transferred and then the departing alcohol
molecule can leave to form a carbocation in a distinct second step. This is the
specific acid-catalyzed mechanism.
2. The acetal can undergo ionization with formation of an alkoxide ion and a
carbocation. The alkoxide is protonated in a second step. This mechanism is
extremely rare, if not impossible, because an alkoxide ion is a poor leaving
group. An alternative mechanism involves general acid catalysis, in which the
proton transfer and the C−O bond rupture occur as a concerted process. The
concerted process need not be perfectly synchronous; that is, proton transfer