Page 662 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 662
644 + +
R 2 COR' R 2 COR'
–
+
+ H + OR' + R'OH
CHAPTER 7
Addition, Condensation
O bond breaking
and Substitution
Reactions of Carbonyl
Compounds
C
R C(OR') 2 R COR'
2
2
+ H + O H bond formation
HOR'
+
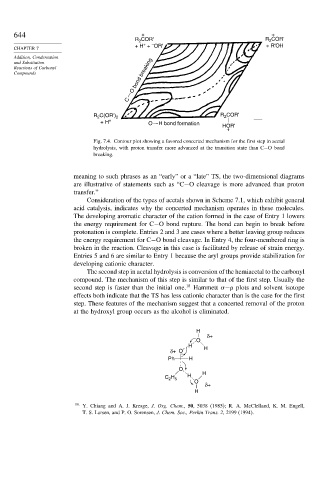
Fig. 7.4. Contour plot showing a favored concerted mechanism for the first step in acetal
hydrolysis, with proton transfer more advanced at the transition state than C−O bond
breaking.
meaning to such phrases as an “early” or a “late” TS, the two-dimensional diagrams
are illustrative of statements such as “C−O cleavage is more advanced than proton
transfer.”
Consideration of the types of acetals shown in Scheme 7.1, which exhibit general
acid catalysis, indicates why the concerted mechanism operates in these molecules.
The developing aromatic character of the cation formed in the case of Entry 1 lowers
the energy requirement for C−O bond rupture. The bond can begin to break before
protonation is complete. Entries 2 and 3 are cases where a better leaving group reduces
the energy requirement for C−O bond cleavage. In Entry 4, the four-membered ring is
broken in the reaction. Cleavage in this case is facilitated by release of strain energy.
Entries 5 and 6 are similar to Entry 1 because the aryl groups provide stabilization for
developing cationic character.
The second step in acetal hydrolysis is conversion of the hemiacetal to the carbonyl
compound. The mechanism of this step is similar to that of the first step. Usually the
second step is faster than the initial one. 18 Hammett − plots and solvent isotope
effects both indicate that the TS has less cationic character than is the case for the first
step. These features of the mechanism suggest that a concerted removal of the proton
at the hydroxyl group occurs as the alcohol is eliminated.
H
δ+
O
H H
δ+ O
Ph H
O
H
C H H
2 5
O
δ+
H
18
Y. Chiang and A. J. Kresge, J. Org. Chem., 50, 5038 (1985); R. A. McClelland, K. M. Engell,
T. S. Larsen, and P. O. Sorensen, J. Chem. Soc., Perkin Trans. 2, 2199 (1994).