Page 666 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 666
648 hydroxide ion on the protonated imine (Step 3). The concentration of both of these
species is pH dependent, but in opposite, compensating, ways. The overall rate is
CHAPTER 7
therefore pH independent in the alkaline range. (Work problem 7.26 to establish that
Addition, Condensation this is so.) Note that in this region the substituent effect is considerably smaller and is
and Substitution
Reactions of Carbonyl in the opposite sense from the acidic portion of the profile. This is due to the enhanced
Compounds basicity of the ERG-substituted imines. At any given pH, more of the protonated imine
is present for the ERG substituents.
The pH-rate profile for the hydrolysis of the N-methylimine of 2-methylpropanal
is shown in Figure 7.6. The curve is similar to that for aromatic ketones with EWG
substituents. The rate increases in the pH range 0–4.5, where decomposition of the
zwitterionic intermediate is rate controlling. In the pH range 4.5–8, the rate decreases
and then levels off. This corresponds to the transformation of the protonated imine to
the less reactive neutral form. Above pH 8, the rate is again constant, as the increase
−
in [ OH] is compensated by the decrease in the amount of protonated imine.
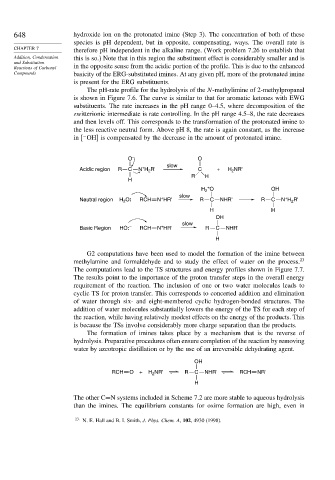
O – O
slow
+
Acidic region R C N H R' C + H NR'
2
2
R H
H
+
H O OH
2
slow
+
+
Neutral region H O: RCH N HR' R C NHR' R C N H R'
2
2
H H
OH
slow
+
Basic Region HO: – RCH N HR' R C NHR'
H
G2 computations have been used to model the formation of the imine between
methylamine and formaldehyde and to study the effect of water on the process. 23
The computations lead to the TS structures and energy profiles shown in Figure 7.7.
The results point to the importance of the proton transfer steps in the overall energy
requirement of the reaction. The inclusion of one or two water molecules leads to
cyclic TS for proton transfer. This corresponds to concerted addition and elimination
of water through six- and eight-membered cyclic hydrogen-bonded structures. The
addition of water molecules substantially lowers the energy of the TS for each step of
the reaction, while having relatively modest effects on the energy of the products. This
is because the TSs involve considerably more charge separation than the products.
The formation of imines takes place by a mechanism that is the reverse of
hydrolysis. Preparative procedures often ensure completion of the reaction by removing
water by azeotropic distillation or by the use of an irreversible dehydrating agent.
OH
RCH O + H NR' R C NHR' RCH NR'
2
H
The other C=N systems included in Scheme 7.2 are more stable to aqueous hydrolysis
than the imines. The equilibrium constants for oxime formation are high, even in
23
N. E. Hall and B. I. Smith, J. Phys. Chem. A, 102, 4930 (1998).