Page 670 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 670
652
–1 –1
CHAPTER 7
Addition, Condensation
and Substitution
log k (sec –1 ) log k obs –2
Reactions of Carbonyl –2
Compounds
–3 –3
5 6 7 8 9 10 11 5 6 7 8 9 10 11 12
pH(pD) pH
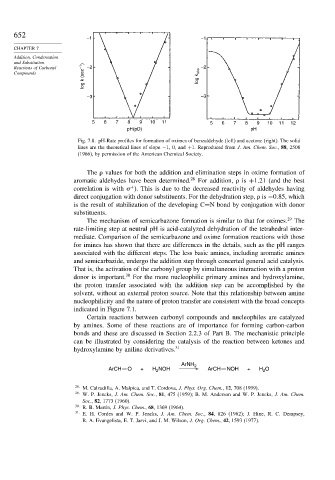
Fig. 7.8. pH-Rate profiles for formation of oximes of benzaldehyde (left) and acetone (right). The solid
lines are the theoretical lines of slope −1, 0, and +1. Reproduced from J. Am. Chem. Soc., 88, 2508
(1966), by permission of the American Chemical Society.
The values for both the addition and elimination steps in oxime formation of
aromatic aldehydes have been determined. 28 For addition, is +1 21 (and the best
correlation is with ). This is due to the decreased reactivity of aldehydes having
+
direct conjugation with donor substituents. For the dehydration step, is −0 85, which
is the result of stabilization of the developing C=N bond by conjugation with donor
substituents.
The mechanism of semicarbazone formation is similar to that for oximes. 29 The
rate-limiting step at neutral pH is acid-catalyzed dehydration of the tetrahedral inter-
mediate. Comparison of the semicarbazone and oxime formation reactions with those
for imines has shown that there are differences in the details, such as the pH ranges
associated with the different steps. The less basic amines, including aromatic amines
and semicarbazide, undergo the addition step through concerted general acid catalysis.
That is, the activation of the carbonyl group by simultaneous interaction with a proton
donor is important. 30 For the more nucleophilic primary amines and hydroxylamine,
the proton transfer associated with the addition step can be accomplished by the
solvent, without an external proton source. Note that this relationship between amine
nucleophilicity and the nature of proton transfer are consistent with the broad concepts
indicated in Figure 7.1.
Certain reactions between carbonyl compounds and nucleophiles are catalyzed
by amines. Some of these reactions are of importance for forming carbon-carbon
bonds and these are discussed in Section 2.2.3 of Part B. The mechanistic principle
can be illustrated by considering the catalysis of the reaction between ketones and
hydroxylamine by aniline derivatives. 31
ArNH 2
ArCH O + H NOH ArCH NOH + H O
2
2
28 M. Calzadilla, A. Malpica, and T. Cordova, J. Phys. Org. Chem., 12, 708 (1999).
29
W. P. Jencks, J. Am. Chem. Soc., 81, 475 (1959); B. M. Anderson and W. P. Jencks, J. Am. Chem.
Soc., 82, 1773 (1960).
30 R. B. Martin, J. Phys. Chem., 68, 1369 (1964).
31
E. H. Cordes and W. P. Jencks, J. Am. Chem. Soc., 84, 826 (1962); J. Hine, R. C. Dempsey,
R. A. Evangelista, E. T. Jarvi, and J. M. Wilson, J. Org. Chem., 42, 1593 (1977).