Page 675 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 675
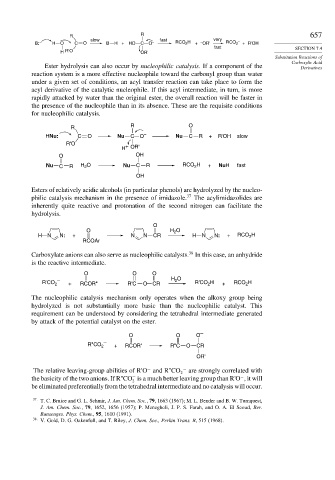
R
R 657
slow fast very –
B: – H O C O B H + HO C O – RCO 2 H + – OR' RCO 2 + R'OH
fast SECTION 7.4
H R'O OR'
Substitution Reactions of
Carboxylic Acid
Ester hydrolysis can also occur by nucleophilic catalysis. If a component of the Derivatives
reaction system is a more effective nucleophile toward the carbonyl group than water
under a given set of conditions, an acyl transfer reaction can take place to form the
acyl derivative of the catalytic nucleophile. If this acyl intermediate, in turn, is more
rapidly attacked by water than the original ester, the overall reaction will be faster in
the presence of the nucleophile than in its absence. These are the requisite conditions
for nucleophilic catalysis.
R R O
HNu: C O Nu C O – Nu C R + R'OH slow
R'O
H + OR'
O OH
Nu C R H O Nu C R RCO H + NuH fast
2
2
OH
Esters of relatively acidic alcohols (in particular phenols) are hydrolyzed by the nucleo-
philic catalysis mechanism in the presence of imidazole. 37 The acylimidazolides are
inherently quite reactive and protonation of the second nitrogen can facilitate the
hydrolysis.
O
O H O
2
H N N: + N N CR H N N: + RCO H
2
RCOAr
38
Carboxylate anions can also serve as nucleophilic catalysts. In this case, an anhydride
is the reactive intermediate.
O O O
– H O
2
2
R'CO 2 + RCOR" R'C O CR R'CO H + RCO H
2
The nucleophilic catalysis mechanism only operates when the alkoxy group being
hydrolyzed is not substantially more basic than the nucleophilic catalyst. This
requirement can be understood by considering the tetrahedral intermediate generated
by attack of the potential catalyst on the ester.
–
O O O
R"CO 2 – + RCOR' R"C O CR
OR'
−
The relative leaving-group abilities of R'O and R''CO 2 − are strongly correlated with
−
the basicity of the two anions. If R''CO is a much better leaving group than R'O , it will
−
2
be eliminated preferentially from the tetrahedral intermediate and no catalysis will occur.
37 T. C. Bruice and G. L. Schmir, J. Am. Chem. Soc., 79, 1663 (1967); M. L. Bender and B. W. Turnquest,
J. Am. Chem. Soc., 79, 1652, 1656 (1957); P. Menegheli, J. P. S. Farah, and O. A. El Seoud, Ber.
Bunsenges. Phys. Chem., 95, 1610 (1991).
38
V. Gold, D. G. Oakenfull, and T. Riley, J. Chem. Soc., Perkin Trans. B, 515 (1968).