Page 678 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 678
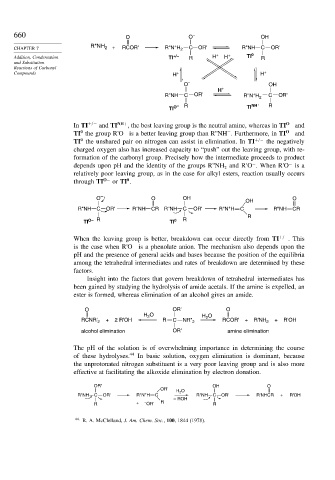
660 O O – OH
R"NH +
CHAPTER 7 2 + RCOR' R"N H 2 C OR' R"NH C OR'
Addition, Condensation TI +/– R H + H + TI 0 R
and Substitution
Reactions of Carbonyl
Compounds H + H +
O – OH
H +
+
R"NH C OR' R"N H 2 C OR'
O – R TI NH + R
TI
In TI +/− and TI NH+ , the best leaving group is the neutral amine, whereas in TI O− and
0
−
−
TI the group R'O is a better leaving group than R''NH . Furthermore, in TI O− and
0
TI the unshared pair on nitrogen can assist in elimination. In TI +/− the negatively
charged oxygen also has increased capacity to “push” out the leaving group, with re-
formation of the carbonyl group. Precisely how the intermediate proceeds to product
depends upon pH and the identity of the groups R''NH and R O . When R'O is a
−
−
2
relatively poor leaving group, as in the case for alkyl esters, reaction usually occurs
0
through TI O− or TI .
O – O OH O
OH
+
R"NH C OR' R"NH CR R"NH C OR' R"N H C R"NH CR
R
TI O– R TI 0 R
When the leaving group is better, breakdown can occur directly from TI +/− . This
−
is the case when R'O is a phenolate anion. The mechanism also depends upon the
pH and the presence of general acids and bases because the position of the equilibria
among the tetrahedral intermediates and rates of breakdown are determined by these
factors.
Insight into the factors that govern breakdown of tetrahedral intermediates has
been gained by studying the hydrolysis of amide acetals. If the amine is expelled, an
ester is formed, whereas elimination of an alcohol gives an amide.
O OR' O
H O H O
2
2
RCNR' 2 + 2 R'OH R C NR" 2 RCOR' + R'NH 2 + R'OH
alcohol elimination OR' amine elimination
The pH of the solution is of overwhelming importance in determining the course
of these hydrolyses. 44 In basic solution, oxygen elimination is dominant, because
the unprotonated nitrogen substituent is a very poor leaving group and is also more
effective at facilitating the alkoxide elimination by electron donation.
OR' OH O
OR' H 2 O
+
R"NH C OR' R"N H C R"NH C OR' R'NHCR + R'OH
– ROH
R + – OR' R R
44
R. A. McClelland, J. Am. Chem. Soc., 100, 1844 (1978).