Page 673 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 673
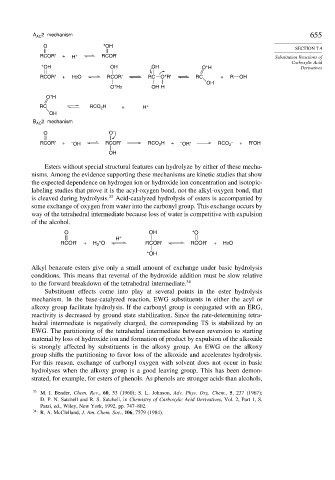
A AC 2 mechanism 655
O + OH
SECTION 7.4
RCOR' + H + RCOR' Substitution Reactions of
Carboxylic Acid
+ OH OH OH O H Derivatives
+
+
RCOR' + H2O RCOR' RC O R' RC + R OH
OH
+
O H2 OH H
+
O H
RC RCO H + H +
2
OH
B AC 2 mechanism
O O –
RCOR' + – OH RCOR' RCO H + – OR' RCO 2 – + R'OH
2
OH
Esters without special structural features can hydrolyze by either of these mecha-
nisms. Among the evidence supporting these mechanisms are kinetic studies that show
the expected dependence on hydrogen ion or hydroxide ion concentration and isotopic-
labeling studies that prove it is the acyl-oxygen bond, not the alkyl-oxygen bond, that
is cleaved during hydrolysis. 33 Acid-catalyzed hydrolysis of esters is accompanied by
some exchange of oxygen from water into the carbonyl group. This exchange occurs by
way of the tetrahedral intermediate because loss of water is competitive with expulsion
of the alcohol.
O OH *O
H +
RCOR' + H *O RCOR' RCOR' + H2O
2
*OH
Alkyl benzoate esters give only a small amount of exchange under basic hydrolysis
conditions. This means that reversal of the hydroxide addition must be slow relative
to the forward breakdown of the tetrahedral intermediate. 34
Substituent effects come into play at several points in the ester hydrolysis
mechanism. In the base-catalyzed reaction, EWG substituents in either the acyl or
alkoxy group facilitate hydrolysis. If the carbonyl group is conjugated with an ERG,
reactivity is decreased by ground state stabilization. Since the rate-determining tetra-
hedral intermediate is negatively charged, the corresponding TS is stabilized by an
EWG. The partitioning of the tetrahedral intermediate between reversion to starting
material by loss of hydroxide ion and formation of product by expulsion of the alkoxide
is strongly affected by substituents in the alkoxy group. An EWG on the alkoxy
group shifts the partitioning to favor loss of the alkoxide and accelerates hydrolysis.
For this reason, exchange of carbonyl oxygen with solvent does not occur in basic
hydrolyses when the alkoxy group is a good leaving group. This has been demon-
strated, for example, for esters of phenols. As phenols are stronger acids than alcohols,
33 M. I. Bender, Chem. Rev., 60, 53 (1960); S. L. Johnson, Adv. Phys. Org. Chem., 5, 237 (1967);
D. P. N. Satchell and R. S. Satchell, in Chemistry of Carboxylic Acid Derivatives, Vol. 2, Part 1, S.
Patai, ed., Wiley, New York, 1992, pp. 747–802.
34
R. A. McClelland, J. Am. Chem. Soc., 106, 7579 (1984).