Page 661 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 661
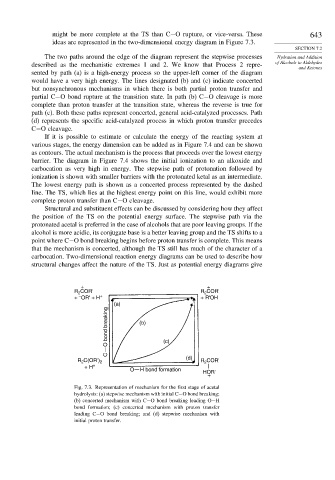
might be more complete at the TS than C−O rupture, or vice-versa. These 643
ideas are represented in the two-dimensional energy diagram in Figure 7.3.
SECTION 7.2
The two paths around the edge of the diagram represent the stepwise processes Hydration and Addition
described as the mechanistic extremes 1 and 2. We know that Process 2 repre- of Alcohols to Aldehydes
and Ketones
sented by path (a) is a high-energy process so the upper-left corner of the diagram
would have a very high energy. The lines designated (b) and (c) indicate concerted
but nonsynchronous mechanisms in which there is both partial proton transfer and
partial C−O bond rupture at the transition state. In path (b) C−O cleavage is more
complete than proton transfer at the transition state, whereas the reverse is true for
path (c). Both these paths represent concerted, general acid-catalyzed processes. Path
(d) represents the specific acid-catalyzed process in which proton transfer precedes
C−O cleavage.
If it is possible to estimate or calculate the energy of the reacting system at
various stages, the energy dimension can be added as in Figure 7.4 and can be shown
as contours. The actual mechanism is the process that proceeds over the lowest energy
barrier. The diagram in Figure 7.4 shows the initial ionization to an alkoxide and
carbocation as very high in energy. The stepwise path of protonation followed by
ionization is shown with smaller barriers with the protonated ketal as an intermediate.
The lowest energy path is shown as a concerted process represented by the dashed
line. The TS, which lies at the highest energy point on this line, would exhibit more
complete proton transfer than C−O cleavage.
Structural and substituent effects can be discussed by considering how they affect
the position of the TS on the potential energy surface. The stepwise path via the
protonated acetal is preferred in the case of alcohols that are poor leaving groups. If the
alcohol is more acidic, its conjugate base is a better leaving group and the TS shifts to a
point where C−O bond breaking begins before proton transfer is complete. This means
that the mechanism is concerted, although the TS still has much of the character of a
carbocation. Two-dimensional reaction energy diagrams can be used to describe how
structural changes affect the nature of the TS. Just as potential energy diagrams give
+ +
R COR' R 2 COR'
2
–
+ OR' + H + + R'OH
(a)
O bond breaking (b)
C (c)
R C(OR') 2 (d) R 2 COR'
2
+ H +
O H bond formation
HOR'
+
Fig. 7.3. Representation of mechanism for the first stage of acetal
hydrolysis: (a) stepwise mechanism with initial C−O bond breaking;
(b) concerted mechanism with C−O bond breaking leading O−H
bond formation; (c) concerted mechanism with proton transfer
leading C−O bond breaking; and (d) stepwise mechanism with
initial proton transfer.