Page 646 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 646
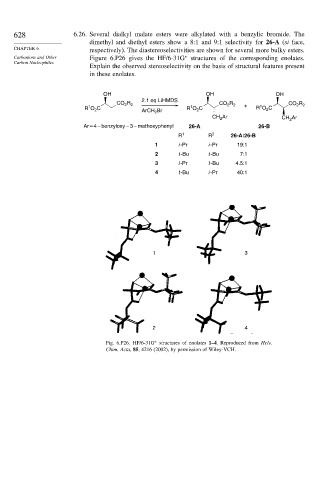
628 6.26. Several dialkyl malate esters were alkylated with a benzylic bromide. The
dimethyl and diethyl esters show a 8:1 and 9:1 selectivity for 26-A (si face,
CHAPTER 6 respectively). The diastereoselectivities are shown for several more bulky esters.
∗
Carbanions and Other Figure 6.P26 gives the HF/6-31G structures of the corresponding enolates.
Carbon Nucleophiles
Explain the observed stereoselectivity on the basis of structural features present
in these enolates.
OH OH OH
2.1 eq LiHMDS
R R
R
CO 2 2 CO 2 2 + CO 2 2
1
1
1
R O C ArCH Br R O C R O C
2
2
2
2
Ar
CH 2 CH Ar
2
Ar = 4 – benzyloxy – 3 – methoxyphenyl 26-A 26-B
R 1 R 2 26-A:26-B
1 i -Pr i -Pr 19:1
2 t -Bu t -Bu 7:1
3 i -Pr t -Bu 4.5:1
4 t -Bu i -Pr 40:1
1 3
2 4
Fig. 6.P26. HF/6-31G structures of enolates 1–4. Reproduced from Helv.
∗
Chim. Acta, 85, 4216 (2002), by permission of Wiley-VCH.