Page 641 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 641
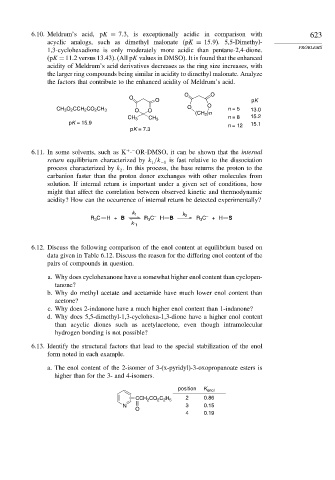
6.10. Meldrum’s acid, pK = 7 3, is exceptionally acidic in comparison with 623
acyclic analogs, such as dimethyl malonate (pK = 15 9). 5,5-Dimethyl-
1,3-cyclohexadione is only moderately more acidic than pentane-2,4-dione. PROBLEMS
(pK = 11 2 versus 13.43). (All pK values in DMSO). It is found that the enhanced
acidity of Meldrum’s acid derivatives decreases as the ring size increases, with
the larger ring compounds being similar in acidity to dimethyl malonate. Analyze
the factors that contribute to the enhanced acidity of Meldrum’s acid.
O O
O
O pK
O O
CH 3 O 2 CCH 2 CO 2 CH 3 O O n = 5 13.0
(CH 2 )n
n = 8 15.2
CH 3 CH 3
pK = 15.9 n = 12 15.1
pK = 7.3
+ −
6.11. In some solvents, such as K · OR-DMSO, it can be shown that the internal
return equilibrium characterized by k /k is fast relative to the dissociation
1 −1
process characterized by k . In this process, the base returns the proton to the
2
carbanion faster than the proton donor exchanges with other molecules from
solution. If internal return is important under a given set of conditions, how
might that affect the correlation between observed kinetic and thermodynamic
acidity? How can the occurrence of internal return be detected experimentally?
k 1 k
R C H+ B R C – H B 2 R C – + H S
3
3
3
k- 1
6.12. Discuss the following comparison of the enol content at equilibrium based on
data given in Table 6.12. Discuss the reason for the differing enol content of the
pairs of compounds in question.
a. Why does cyclohexanone have a somewhat higher enol content than cyclopen-
tanone?
b. Why do methyl acetate and acetamide have much lower enol content than
acetone?
c. Why does 2-indanone have a much higher enol content than 1-indanone?
d. Why does 5,5-dimethyl-1,3-cyclohexa-1,3-dione have a higher enol content
than acyclic diones such as acetylacetone, even though intramolecular
hydrogen bonding is not possible?
6.13. Identify the structural factors that lead to the special stabilization of the enol
form noted in each example.
a. The enol content of the 2-isomer of 3-(x-pyridyl)-3-oxopropanoate esters is
higher than for the 3- and 4-isomers.
position K enol
CCH CO C H 2 0.86
2
2 2 5
N 3 0.15
O
4 0.19