Page 642 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 642
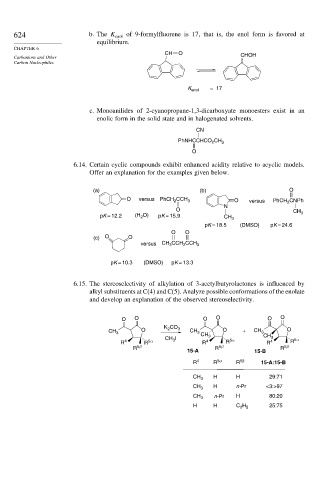
624 b. The K enol of 9-formylfluorene is 17, that is, the enol form is favored at
equilibrium.
CHAPTER 6
CH O CHOH
Carbanions and Other
Carbon Nucleophiles
= 17
K enol
c. Monoanilides of 2-cyanopropane-1,3-dicarboxyate monoesters exist in an
enolic form in the solid state and in halogenated solvents.
CN
PhNHCCHCO CH 3
2
O
6.14. Certain cyclic compounds exhibit enhanced acidity relative to acyclic models.
Offer an explanation for the examples given below.
(a) (b) O
O versus PhCH CCH 3 O versus PhCH CNPh
2
2
N
O
CH 3
pK = 12.2 (H O) pK = 15.9 CH 3
2
pK = 18.5 (DMSO) pK = 24.6
O O
(c) O O
versus CH CCH CCH 3
3
2
pK = 10.3 (DMSO) pK = 13.3
6.15. The stereoselectivity of alkylation of 3-acetylbutyrolactones is influenced by
alkyl substituents at C(4) and C(5). Analyze possible conformations of the enolate
and develop an explanation of the observed stereoselectivity.
O O O O O O
K CO
O 2 3 O CH O
CH 3 CH 3 CH 3 + 3 CH
3
R 4 R 5α CH I R 4 R 5α R 4 3 R 5α
R 5β R 5β R 5β
15-A 15-B
R 4 R 5α R 5β 15-A:15-B
CH 3 H H 29:71
CH 3 H n-Pr <3:>97
CH 3 n-Pr H 80:20
H H C H 25:75
2 5