Page 623 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 623
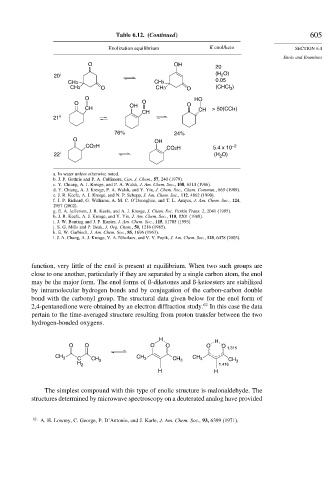
Table 6.12. (Continued) 605
Enolization equilibrium K enol/keto SECTION 6.4
Enols and Enamines
O OH
20
20 j (H 2 O)
0.05
CH3 CH3
CH3 O CH3 O (CHCl )
3
O HO
O OH O O
CH CH > 50(CCl4)
CH
21 k
76% 24%
O OH
CO2H CO2H 5.4 x 10 –2
22 l (H O)
2
a. In water unless otherwise noted.
b. J. P. Guthrie and P. A. Cullimore, Can. J. Chem., 57, 240 (1979).
c. Y. Chiang, A. J. Kresge, and P. A. Walsh, J. Am. Chem. Soc., 108, 6314 (1986).
d. Y. Chiang, A. J. Kresge, P. A. Walsh, and Y. Yin, J. Chem. Soc., Chem. Commun., 869 (1989).
e. J. R. Keefe, A. J. Kresge, and N. P. Schepp, J. Am. Chem. Soc., 112, 4862 (1990).
f. J. P. Richard, G. Williams, A. M. C. O’Donoghue, and T. L. Amyes, J. Am. Chem. Soc., 124,
2957 (2002).
g. E. A. Jefferson, J. R. Keefe, and A. J. Kresge, J. Chem. Soc. Perkin Trans. 2, 2041 (1995).
h. J. R. Keefe, A. J. Kresge, and Y. Yin, J. Am. Chem. Soc., 110, 8201 (1988).
i. J. W. Bunting and J. P. Kanter, J. Am. Chem. Soc., 115, 11705 (1993).
j. S. G. Mills and P. Beak, J. Org. Chem., 50, 1216 (1985).
k. E. W. Garbisch, J. Am. Chem. Soc., 85, 1696 (1963).
l. J. A. Chang, A. J. Kresge, V. A. Nikolaev, and V. V. Popik, J. Am. Chem. Soc., 125, 6478 (2003).
function, very little of the enol is present at equilibrium. When two such groups are
close to one another, particularly if they are separated by a single carbon atom, the enol
may be the major form. The enol forms of ß-diketones and ß-ketoesters are stabilized
by intramolecular hydrogen bonds and by conjugation of the carbon-carbon double
bond with the carbonyl group. The structural data given below for the enol form of
62
2,4-pentanedione were obtained by an electron diffraction study. In this case the data
pertain to the time-averaged structure resulting from proton transfer between the two
hydrogen-bonded oxygens.
H H
O O O O O O 1.315
CH 3 C CH 3 CH 3 CH 3 CH 3
H 2 1.416 CH 3
H H
The simplest compound with this type of enolic structure is malonaldehyde. The
structures determined by microwave spectroscopy on a deuterated analog have provided
62
A. H. Lowrey, C. George, P. D’Antonio, and J. Karle, J. Am. Chem. Soc., 93, 6399 (1971).