Page 614 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 614
596 controlled conditions. In general, the more highly substituted enolate is the preferred
isomer, but if the alkyl groups are sufficiently branched as to interfere with
CHAPTER 6 solvation of the enolate there are exceptions. This factor, along with CH /CH
3 3
Carbanions and Other repulsion, presumably accounts for the higher stability of the less-substituted enolate
Carbon Nucleophiles
from 3-methyl-2-butanone (Entry 2). The acidifying effect of an adjacent phenyl
group outweighs steric effects in the case of 1-phenyl-2-propanone, and as a result
the conjugated enolate is favored by both kinetic and thermodynamic conditions
(Entry 5).
The synthetic importance of the LDA and LiHMDS type of deprotonation has
led to studies of enolate composition under various conditions. Deprotonation of
2-pentanone was examined with LDA in THF, with and without HMPA. C(1)-
deprotonation was favored under both conditions, but the Z:E ratio for C(3) deproto-
nation was sensitive to the presence of HMPA (0.75 M . 46 More Z-enolate is formed
when HMPA is present.
Conditions Ratio C(1):C(3) deprotonation Ratio Z:E for C(3) deprotonation
0 C, THF alone 7.9 0.20
−60 C, THF alone 7.1 0.15
0 C THF −HMPA 8.0 1.0
−60 C THF −HMPA 5.6 3.1
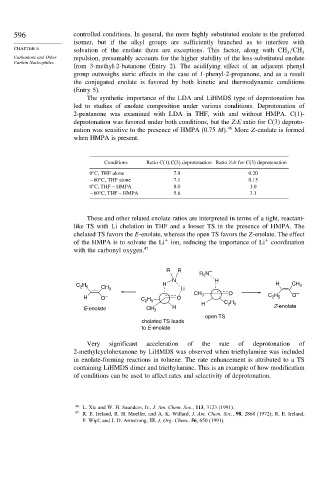
These and other related enolate ratios are interpreted in terms of a tight, reactant-
like TS with Li chelation in THF and a looser TS in the presence of HMPA. The
chelated TS favors the E-enolate, whereas the open TS favors the Z-enolate. The effect
+
+
of the HMPA is to solvate the Li ion, reducing the importance of Li coordination
with the carbonyl oxygen. 47
R R –
R N
2
N H
C H CH 3 H Li H CH 3
2 5
CH 3 O O –
H O – C H O C H
2 5
2 5
H C H
2 5
E-enolate CH 3 H Z-enolate
open TS
chelated TS leads
to E-enolate
Very significant acceleration of the rate of deprotonation of
2-methylcyclohexanone by LiHMDS was observed when triethylamine was included
in enolate-forming reactions in toluene. The rate enhancement is attributed to a TS
containing LiHMDS dimer and triethylamine. This is an example of how modification
of conditions can be used to affect rates and selectivity of deprotonation.
46 L. Xie and W. H. Saunders, Jr., J. Am. Chem. Soc., 113, 3123 (1991).
47
R. E. Ireland, R. H. Mueller, and A. K. Willard, J. Am. Chem. Soc., 98, 2868 (1972); R. E. Ireland,
P. Wipf, and J. D. Armstrong, III, J. Org. Chem., 56, 650 (1991).