Page 610 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 610
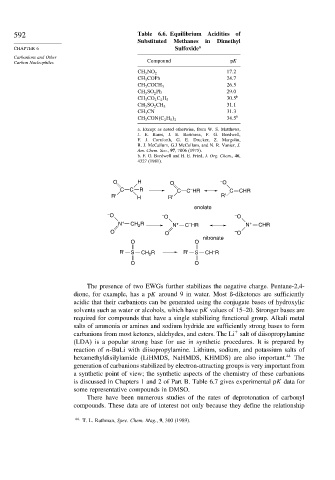
592 Table 6.6. Equilibrium Acidities of
Substituted Methanes in Dimethyl
CHAPTER 6 Sulfoxide a
Carbanions and Other
Carbon Nucleophiles Compound pK
17 2
CH 3 NO 2
CH 3 COPh 24 7
26 5
CH 3 COCH 3
CH 3 SO 2 Ph 29 0
30 5 b
CH 3 CO 2 C 2 H 5
31 1
CH 3 SO 2 CH 3
CH 3 CN 31 3
34 5 b
CH 3 CON C 2 H 5 2
a. Except as noted otherwise, from W. S. Matthews,
J. E. Bares, J. E. Bartmess, F. G. Bordwell,
F. J. Cornforth, G. E. Drucker, Z. Margolin,
R. J. McCallum, G.J McCollum, and N. R. Vanier, J.
Am. Chem. Soc., 97, 7006 (1975).
b. F. G. Bordwell and H. E. Fried, J. Org. Chem., 46,
4327 (1981).
O H O – O
C C R C C HR C CHR
–
R' H R' R'
enolate
– O – O – O
N + CH 2 R N + C HR N + CHR
–
O O – O
nitronate
O O
–
R' S CH R R' S CH R
2
O O
The presence of two EWGs further stabilizes the negative charge. Pentane-2,4-
dione, for example, has a pK around 9 in water. Most ß-diketones are sufficiently
acidic that their carbanions can be generated using the conjugate bases of hydroxylic
solvents such as water or alcohols, which have pK values of 15–20. Stronger bases are
required for compounds that have a single stabilizing functional group. Alkali metal
salts of ammonia or amines and sodium hydride are sufficiently strong bases to form
+
carbanions from most ketones, aldehydes, and esters. The Li salt of diisopropylamine
(LDA) is a popular strong base for use in synthetic procedures. It is prepared by
reaction of n-BuLi with diisopropylamine. Lithium, sodium, and potassium salts of
hexamethyldisilylamide (LiHMDS, NaHMDS, KHMDS) are also important. 44 The
generation of carbanions stabilized by electron-attracting groups is very important from
a synthetic point of view; the synthetic aspects of the chemistry of these carbanions
is discussed in Chapters 1 and 2 of Part B. Table 6.7 gives experimental pK data for
some representative compounds in DMSO.
There have been numerous studies of the rates of deprotonation of carbonyl
compounds. These data are of interest not only because they define the relationship
44
T. L. Rathman, Spec. Chem. Mag., 9, 300 (1989).