Page 263 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 263
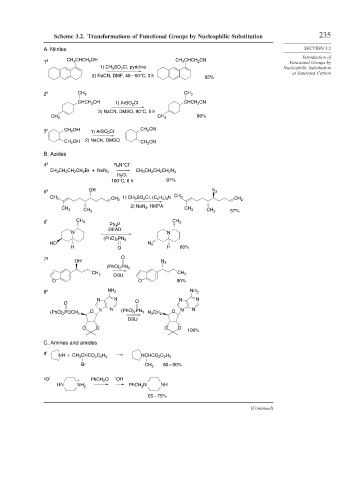
Scheme 3.2. Transformations of Functional Groups by Nucleophilic Substitution 235
A. Nitriles SECTION 3.2
Introduction of
1 a CH 3 CHCH 2 OH CH 3 CHCH 2 CN Functional Groups by
1) CH 3 SO 2 Cl, pyridine Nucleophilic Substitution
at Saturated Carbon
2) NaCN, DMF, 40 – 60°C, 3 h 85%
2 b CH 3 CH 3
CHCH 2 OH 1) ArSO 2 Cl CHCH 2 CN
2) NaCN, DMSO, 90°C, 5 h
80%
CH 3 CH 3
3 c CH 2 OH 1) ArSO 2 Cl CH 2 CN
CH 2 OH 2) NaCN, DMSO CH 2 CN
B. Azides
+
4 d R 4 N Cl –
CH 3 CH 2 CH 2 CH 2 Br + NaN 3 CH 3 CH 2 CH 2 CH 2 N 3
H 2 O,
100°C, 6 h 97%
5 e OH N 3
1) CH 3 SO 2 Cl, (C 2 H 5 ) 3 N CH 3
CH 3
CH 2 CH 2
2) NaN 3 , HMPA
CH 3 CH 3 57%
CH 3 CH 3
6 f CH 3 CH 3
Ph 3 P
DEAD
N N
(PhO) 2 PN 3
HO N 3
H O H 60%
7 g O
OH N 3
(PhO) 2 PN 3
CH 3 CH 3
DBU
O O 90%
8 h NH 2 NH 2
N N O N N
O
O N N O N N
(PhO) 2 POCH 2 (PhO) 2 PN 3 N 3 CH 2
DBU
O O O O
100%
C. Amines and amides
9 i NH + CH 3 CHCO 2 C 2 H 5 NCHCO 2 C 2 H 5
Br CH 3 80 – 90%
10 j + PhCH 2 Cl – OH
HN NH 2 PhCH 2 N NH
65 – 75%
(Continued)