Page 264 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 264
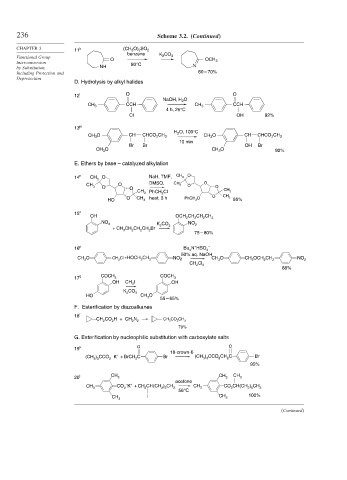
236 Scheme 3.2. (Continued)
CHAPTER 3 11 k (CH 3 O) 2 SO 2
benzene
Functional Group O K 2 CO 3
Interconversion 80°C OCH 3
by Substitution, NH N
Including Protection and 60 – 70%
Deprotection
D. Hydrolysis by alkyl halides
12 l O O
NaOH, H 2 O
CCH CCH
CH 3 CH 3
4 h, 25°C
Cl OH 92%
13 m
H 2 O, 100°C
CH 3 O CH CHCO 2 CH 3 CH 3 O CH CHCO 2 CH 3
10 min
Br Br OH Br
CH 3 O CH 3 O 92%
E. Ethers by base – catalyzed alkylation
14 n CH O NaH, TMF, CH 3 O
3
CH O DMSO, CH 3 O O
3 O O O
CH 3 PhCH Cl CH 3
2
O CH heat, 3 h PhCH 2 O O CH 3
HO 3 95%
15 o
OH OCH CH CH CH
2 2 2 3
NO 2 K CO NO 2
+ CH CH CH CH Br 2 3
2
2
3
2
75 – 80%
p + –
16 Bu N HSO 4
4
50% aq. NaOH
CH O CH 2 Cl +HOCH CH NO 2 CH O CH OCH CH NO
3 2 2 3 2 2 2 2
CH Cl
2 2
88%
17 q COCH 3 COCH 3
OH CH I OH
3
K CO 3
2
HO CH O
3
55 – 65%
F. Esterification by diazoalkanes
18 r
CH CO H + CH N 2 CH 2 CO 2 CH 3
2
2
2
79%
G. Esterification by nucleophilic substitution with carboxylate salts
19 s O O
18-crown-6
+
(CH ) CCO – K + BrCH C Br (CH ) CCO CH C Br
3 3 2 2 3 3 2 2
95%
20 t CH 3 CH 3 CH 3
acetone
– +
CH CO K + CH CH(CH ) CH CH CO CH(CH ) CH
3 2 3 2 5 3 3 2 2 5 3
56°C
CH 3 I CH 3 100%
(Continued)