Page 332 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 332
49
50
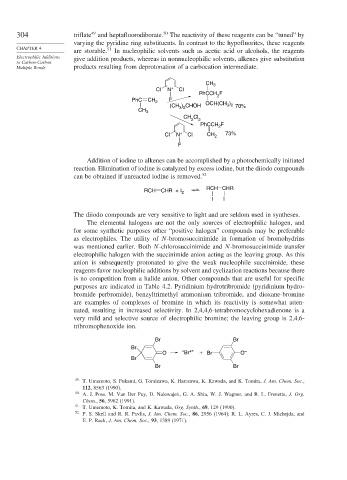
304 triflate and heptafluorodiborate. The reactivity of these reagents can be “tuned” by
varying the pyridine ring substituents. In contrast to the hypofluorites, these reagents
CHAPTER 4 51
are storable. In nucleophilic solvents such as acetic acid or alcohols, the reagents
Electrophilic Additions give addition products, whereas in nonnucleophilic solvents, alkenes give substitution
to Carbon-Carbon
Multiple Bonds products resulting from deprotonation of a carbocation intermediate.
CH 3
Cl N + Cl
PhCCH F
2
PhC CH 2 F
(CH ) CHOH OCH(CH )
3 2 70%
3 2
CH 3
CH Cl
2 2
PhCCH F
2
Cl N + Cl CH 2 73%
F
Addition of iodine to alkenes can be accomplished by a photochemically initiated
reaction. Elimination of iodine is catalyzed by excess iodine, but the diiodo compounds
can be obtained if unreacted iodine is removed. 52
RCH CHR
RCH CHR + I 2
I I
The diiodo compounds are very sensitive to light and are seldom used in syntheses.
The elemental halogens are not the only sources of electrophilic halogen, and
for some synthetic purposes other “positive halogen” compounds may be preferable
as electrophiles. The utility of N-bromosuccinimide in formation of bromohydrins
was mentioned earlier. Both N-chlorosuccinimide and N-bromosuccinimide transfer
electrophilic halogen with the succinimide anion acting as the leaving group. As this
anion is subsequently protonated to give the weak nucleophile succinimide, these
reagents favor nucleophilic additions by solvent and cyclization reactions because there
is no competition from a halide anion. Other compounds that are useful for specific
purposes are indicated in Table 4.2. Pyridinium hydrotribromide (pyridinium hydro-
bromide perbromide), benzyltrimethyl ammonium tribromide, and dioxane-bromine
are examples of complexes of bromine in which its reactivity is somewhat atten-
uated, resulting in increased selectivity. In 2,4,4,6-tetrabromocyclohexadienone is a
very mild and selective source of electrophilic bromine; the leaving group is 2,4,6-
tribromophenoxide ion.
Br Br
Br
+
O “Br ” + Br O –
Br
Br Br
49
T. Umemoto, S. Fukami, G. Tomizawa, K. Harasawa, K. Kawada, and K. Tomita, J. Am. Chem. Soc.,
112, 8563 (1990).
50 A. J. Poss. M. Van Der Puy, D. Nalewajek, G. A. Shia, W. J. Wagner, and R. L. Frenette, J. Org.
Chem., 56, 5962 (1991).
51 T. Umemoto, K. Tomita, and K. Kawada, Org. Synth., 69, 129 (1990).
52
P. S. Skell and R. R. Pavlis, J. Am. Chem. Soc., 86, 2956 (1964); R. L. Ayres, C. J. Michejda, and
E. P. Rack, J. Am. Chem. Soc., 93, 1389 (1971).