Page 327 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 327
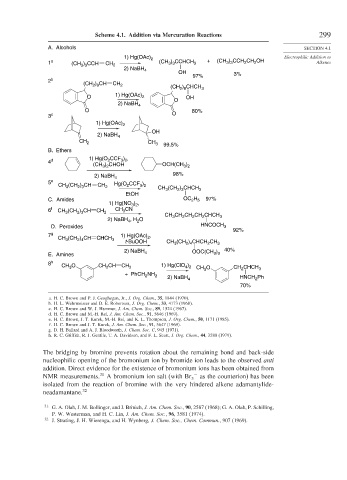
Scheme 4.1. Addition via Mercuration Reactions 299
A. Alcohols SECTION 4.1
1) Hg(OAc) 2 Electrophilic Addition to
) CCHCH
1 a (CH ) CCH CH 2 (CH 3 3 3 + (CH ) CCH CH OH Alkenes
3 3
2
2
3 3
2) NaBH 4
OH 3%
97%
2 b
(CH ) CH CH 2 (CH ) CHCH 3
2 8
2 8
O 1) Hg(OAc) 2 O OH
2) NaBH 4
O 80%
3 c O
1) Hg(OAc) 2
OH
2) NaBH 4
CH 2 CH 3 99.5%
B. Ethers
1) Hg(O CCF ) ,
4 d 2 3 2
(CH ) CHOH OCH(CH )
3 2
3 2
98%
2) NaBH 4
5 e Hg(O CCF )
CH (CH ) CH CH 2 2 3 2 CH (CH ) CHCH 3
2 3
3
2 3
3
EtOH
C. Amides OC H 97%
2 5
1) Hg(NO 3 ) 2 ,
6 f CH (CH ) CH CH 2 CH 3 CN
3
2 3
CH CH CH CH CHCH 3
2
2
3
2
2) NaBH , H O
4
2
D. Peroxides HNCOCH 3
92%
7 g 1) Hg(OAc) ,
CH (CH ) CH CHCH 3 2
2 4
3
t-BuOOH CH (CH ) CHCH CH 3
3
2
2 4
2) NaBH 4 OOC(CH ) 40%
E. Amines 3 3
8 h CH O CH CH CH 2 1) Hg(ClO ) CH O CH 2 CHCH 3
4 2
3
2
+ PhCH NH 2 2) NaBH 4 3 HNCH Ph
2
2
70%
a. H. C. Brown and P. J. Geoghegan, Jr., J. Org. Chem., 35, 1844 (1970).
b. H. L. Wehrmeister and D. E. Robertson, J. Org. Chem., 33, 4173 (1968).
c. H. C. Brown and W. J. Hammar, J. Am. Chem. Soc., 89, 1524 (1967).
d. H. C. Brown and M.-H. Rei, J. Am. Chem. Soc., 91, 5646 (1969).
e. H. C. Brown, J. T. Kurek, M.-H. Rei, and K. L. Thompson, J. Org. Chem., 50, 1171 (1985).
f. H. C. Brown and J. T. Kurek, J. Am. Chem. Soc., 91, 5647 (1969).
g. D. H. Ballard and A. J. Bloodworth, J. Chem. Soc. C, 945 (1971).
h. R. C. Griffith, R. J. Gentile, T. A. Davidson, and F. L. Scott, J. Org. Chem., 44, 3580 (1979).
The bridging by bromine prevents rotation about the remaining bond and back-side
nucleophilic opening of the bromonium ion by bromide ion leads to the observed anti
addition. Direct evidence for the existence of bromonium ions has been obtained from
NMR measurements. 31 A bromonium ion salt (with Br 3 − as the counterion) has been
isolated from the reaction of bromine with the very hindered alkene adamantylide-
neadamantane. 32
31 G. A. Olah, J. M. Bollinger, and J. Brinich, J. Am. Chem. Soc., 90, 2587 (1968); G. A. Olah, P. Schilling,
P. W. Westerman, and H. C. Lin, J. Am. Chem. Soc., 96, 3581 (1974).
32
J. Strating, J. H. Wierenga, and H. Wynberg, J. Chem. Soc., Chem. Commun., 907 (1969).