Page 325 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 325
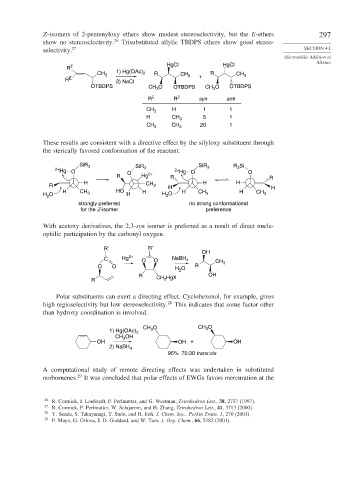
Z-isomers of 2-pentenyloxy ethers show modest stereoselectivity, but the E-ethers 297
show no stereoselectivity. 26 Trisubstituted allylic TBDPS ethers show good stereo-
selectivity. 27 SECTION 4.1
Electrophilic Addition to
Alkenes
HgCl
HgCl
R Z
1) Hg(OAc) 2 R
CH 3 R CH 3 + CH 3
R E 2) NaCl
OTBDPS CH O OTBDPS CH 3 O OTBDPS
3
R E R Z syn anti
CH 3 H 1 1
H CH 3 5 1
CH 3 CH 3 20 1
These results are consistent with a directive effect by the silyloxy substituent through
the sterically favored conformation of the reactant.
SiR 3 SiR SiR R Si
2+ Hg O 3 2+ Hg O 3 3 O
R O Hg 2+ R R
H H H
R CH 3 H H
H CH HO H H H CH
H O 3 H H 2 O CH 3 3
2
strongly preferred no strong conformational
for the Z-isomer preference
With acetoxy derivatives, the 2,3-syn isomer is preferred as a result of direct nucle-
ophilic participation by the carbonyl oxygen.
R′ R′
OH
C Hg 2+ O + O NaBH 4 CH 3
O O H O R
2
R CH HgX OH
R 2
Polar substituents can exert a directing effect. Cyclohexenol, for example, gives
high regioselectivity but low stereoselectivity. 28 This indicates that some factor other
than hydroxy coordination is involved.
O CH O
1) Hg(OAc) 2 CH 3 3
CH OH
3
OH OH + OH
2) NaBH 4
95% 70:30 trans:cis
A computational study of remote directing effects was undertaken in substituted
norbornenes. 29 It was concluded that polar effects of EWGs favors mercuration at the
26
R. Cormick, J. Loefstedt, P. Perlmutter, and G. Westman, Tetrahedron Lett., 38, 2737 (1997).
27
R. Cormick, P. Perlmutter, W. Selajarern, and H. Zhang, Tetrahedron Lett., 41, 3713 (2000).
28 Y. Senda, S. Takayanagi, T. Sudo, and H. Itoh, J. Chem. Soc., Perkin Trans. 1, 270 (2001).
29
P. Mayo, G. Orlova, J. D. Goddard, and W. Tam, J. Org. Chem., 66, 5182 (2001).