Page 324 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 324
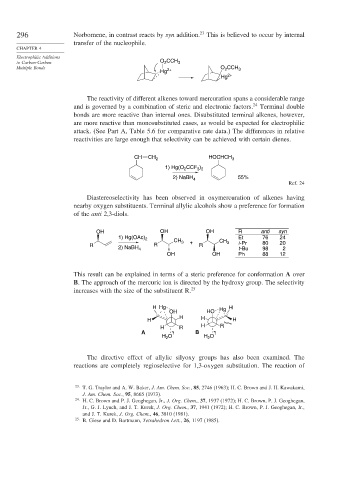
296 Norbornene, in contrast reacts by syn addition. 23 This is believed to occur by internal
transfer of the nucleophile.
CHAPTER 4
Electrophilic Additions
to Carbon-Carbon O CCH 3
2
Multiple Bonds 2+ O CCH 3
2
Hg
Hg 2+
The reactivity of different alkenes toward mercuration spans a considerable range
and is governed by a combination of steric and electronic factors. 24 Terminal double
bonds are more reactive than internal ones. Disubstituted terminal alkenes, however,
are more reactive than monosubstituted cases, as would be expected for electrophilic
attack. (See Part A, Table 5.6 for comparative rate data.) The differences in relative
reactivities are large enough that selectivity can be achieved with certain dienes.
CH CH 2 HOCHCH 3
1) Hg(O CCF )
3 2
2
2) NaBH 4 55%
Ref. 24
Diastereoselectivity has been observed in oxymercuration of alkenes having
nearby oxygen substituents. Terminal allylic alcohols show a preference for formation
of the anti 2,3-diols.
OH OH OH R anti syn
1) Hg(OAc) 2 CH CH Et 76 24
R R 3 + R 3 i -Pr 80 20
2) NaBH 4 t -Bu 98 2
OH OH Ph 88 12
This result can be explained in terms of a steric preference for conformation A over
B. The approach of the mercuric ion is directed by the hydroxy group. The selectivity
increases with the size of the substituent R. 25
H Hg Hg H
OH HO
H H
H H
H R H R
A B
H O H O
2
2
The directive effect of allylic silyoxy groups has also been examined. The
reactions are completely regioselective for 1,3-oxygen substitution. The reaction of
23
T. G. Traylor and A. W. Baker, J. Am. Chem. Soc., 85, 2746 (1963); H. C. Brown and J. H. Kawakami,
J. Am. Chem. Soc., 95, 8665 (1973).
24 H. C. Brown and P. J. Geoghegan, Jr., J. Org. Chem., 37, 1937 (1972); H. C. Brown, P. J. Geoghegan,
Jr., G. J. Lynch, and J. T. Kurek, J. Org. Chem., 37, 1941 (1972); H. C. Brown, P. J. Geoghegan, Jr.,
and J. T. Kurek, J. Org. Chem., 46, 3810 (1981).
25
B. Giese and D. Bartmann, Tetrahedron Lett., 26, 1197 (1985).