Page 366 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 366
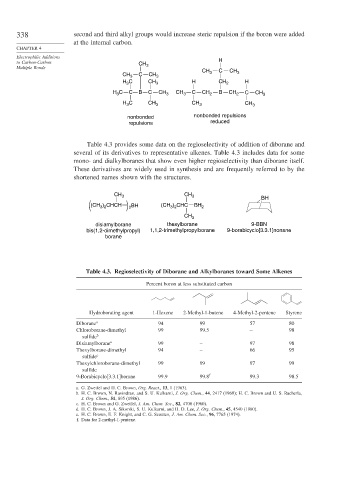
338 second and third alkyl groups would increase steric repulsion if the boron were added
at the internal carbon.
CHAPTER 4
Electrophilic Additions
to Carbon-Carbon CH H
Multiple Bonds 3
CH 3 C CH 3
CH 3 C CH 3
H C CH 3 H CH 2 H
3
C C B C CH CH C CH B C
H 3 3 3 2 CH 2 CH 3
C CH CH CH
H 3 3 3 3
nonbonded nonbonded repulsions
repulsions reduced
Table 4.3 provides some data on the regioselectivity of addition of diborane and
several of its derivatives to representative alkenes. Table 4.3 includes data for some
mono- and dialkylboranes that show even higher regioselectivity than diborane itself.
These derivatives are widely used in synthesis and are frequently referred to by the
shortened names shown with the structures.
CH 3 CH 3
BH
(CH ) CHCH 2 BH (CH ) CHC BH 2
3 2
3 2
CH 3
disiamylborane thexylborane 9-BBN
bis(1,2-dimethylpropyl) 1,1,2-trimethylpropylborane 9-borabicyclo[3.3.1]nonane
borane
Table 4.3. Regioselectivity of Diborane and Alkylboranes toward Some Alkenes
Percent boron at less substituted carbon
Hydroborating agent 1-Hexene 2-Methyl-1-butene 4-Methyl-2-pentene Styrene
Diborane a 94 99 57 80
Chloroborane-dimethyl 99 99.5 − 98
sulfide b
Disiamylborane a 99 – 97 98
Thexylborane-dimethyl 94 – 66 95
sulfide c
Thexylchloroborane-dimethyl 99 99 97 99
sulfide
9-Borabicyclo[3.3.1]borane 99 9 99.8 f 99 3 98 5
a. G. Zweifel and H. C. Brown, Org. React., 13, 1 (1963).
b. H. C. Brown, N. Ravindran, and S. U. Kulkarni, J. Org. Chem., 44, 2417 (1969); H. C. Brown and U. S. Racherla,
J. Org. Chem., 51, 895 (1986).
c. H. C. Brown and G. Zweifel, J. Am. Chem. Soc., 82, 4708 (1960).
d. H. C. Brown, J. A. Sikorski, S. U. Kulkarni, and H. D. Lee, J. Org. Chem., 45, 4540 (1980).
e. H. C. Brown, E. F. Knight, and C. G. Scouten, J. Am. Chem. Soc., 96, 7765 (1974).
f. Data for 2-methyl-1-pentene.