Page 364 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 364
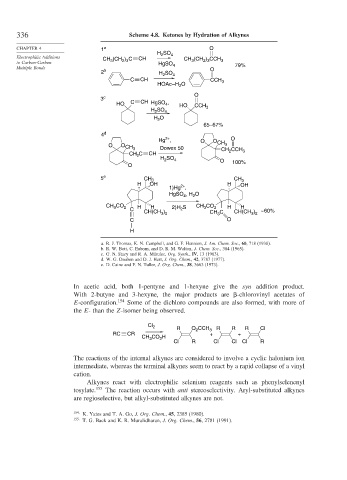
336 Scheme 4.8. Ketones by Hydration of Alkynes
CHAPTER 4 1 a O
H 2 SO 4
Electrophilic Additions CH (CH ) C CH CH (CH ) CCH
to Carbon-Carbon 3 2 3 HgSO 3 2 3 3 79%
Multiple Bonds 4 O
2 b H SO 4
2
C CH CCH 3
HOAc–H O
2
O
3 c
HO C CH HgSO , HO CCH
4
H SO 4 3
2
H O
2
65–67%
4 d
2+
Hg , O O O
O O CH 3 Dowex 50 CH 3
CH CCH
CH C CH 2 3
2
H SO 4 O
2
O 100%
5 e CH 3 CH 3
H OH H OH
2+
1)Hg ,
HgSO 4 , H O
2
CH CO 2 H H 2)H S CH CO 2 H H
3
3
C CH(CH ) 2 CH C CH(CH ) ~60%
3 2
3
3 2
C O
H
a. R. J. Thomas, K. N. Campbell, and G. F. Hennion, J. Am. Chem. Soc., 60, 718 (1938).
b. R. W. Bott, C. Eaborn, and D. R. M. Walton, J. Chem. Soc., 384 (1965).
c. G. N. Stacy and R. A. Mikulec, Org. Synth., IV, 13 (1963).
d. W. G. Dauben and D. J. Hart, J. Org. Chem., 42, 3787 (1977).
e. D. Caine and F. N. Tuller, J. Org. Chem., 38, 3663 (1973).
In acetic acid, both 1-pentyne and 1-hexyne give the syn addition product.
With 2-butyne and 3-hexyne, the major products are -chlorovinyl acetates of
E-configuration. 154 Some of the dichloro compounds are also formed, with more of
the E- than the Z-isomer being observed.
Cl 2
R O CCH 3 R R R Cl
2
RC CR + +
CH CO H
3
2
Cl R Cl Cl Cl R
The reactions of the internal alkynes are considered to involve a cyclic halonium ion
intermediate, whereas the terminal alkynes seem to react by a rapid collapse of a vinyl
cation.
Alkynes react with electrophilic selenium reagents such as phenylselenenyl
tosylate. 155 The reaction occurs with anti stereoselectivity. Aryl-substituted alkynes
are regioselective, but alkyl-substituted alkynes are not.
154 K. Yates and T. A. Go, J. Org. Chem., 45, 2385 (1980).
155
T. G. Back and K. R. Muralidharan, J. Org. Chem., 56, 2781 (1991).