Page 360 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 360
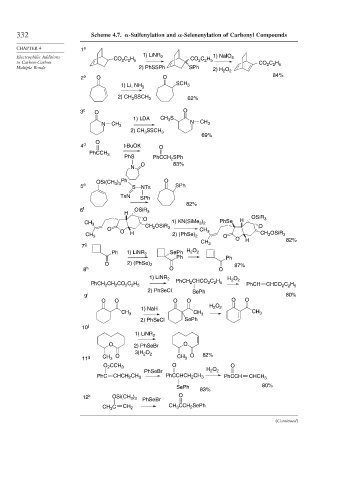
332 Scheme 4.7. -Sulfenylation and -Selenenylation of Carbonyl Compounds
CHAPTER 4 1 a
1) LiNR 1) NaIO
Electrophilic Additions CO C H 2 CO C H 4
to Carbon-Carbon 2 2 5 2 2 5 CO C H
2 2 5
Multiple Bonds 2) PhSSPh SPh 2) H O 2
2
2 b O O 84%
1) Li, NH 3 SCH 3
2) CH SSCH 3 62%
3
3 c O O
1) LDA CH S
3
N CH 3 N CH 3
2) CH SSCH 3
3
69%
O
4 d t-BuOK O
PhCCH 3
PhS PhCCH 2 SPh
O 83%
N
OSi(CH ) Ph O
3 3
5 e S NTs SPh
TsN SPh
82%
6 f OSiR 3
H
O H OSiR 3
CH 3 1) KN(SiMe ) PhSe
3 2
O CH 2 OSiR 3 CH O
CH 3 O H 2) (PhSe) 2 3 O CH 2 OSiR 3
O H 82%
7 g CH 3
2
Ph 1) LiNR 2 SePh H O 2
Ph Ph
O 2) (PhSe) 2 87%
8 h O O
1) LiNR 2 PhCH CHCO C H H O 2
2
PhCH CH CO C H 2 2 2 5 PhCH CHCO C H
2
2 2 5
2 2 5
2
2) PhSeCl SePh
9 i 80%
O O O O O O
H O
1) NaH 2 2
CH 3 CH 3 CH 3
2) PhSeCl SePh
10 j
1) LiNR 2
O 2) PhSeBr O
3)H O 2 82%
2
3
3
11 g CH O CH O
O CCH 3 O O
2
PhSeBr H O 2
2
PhC CHCH 2 CH 3 PhCCHCH CH 3 PhCCH CHCH 3
2
SePh 80%
83%
12 k OSi(CH ) PhSeBr O
3 3
3
CH 3 C CH 2 CH CCH 2 SePh
(Continued)