Page 356 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 356
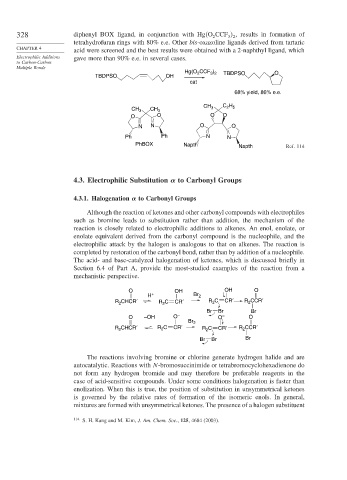
328 diphenyl BOX ligand, in conjunction with Hg O CCF , results in formation of
3 2
2
tetrahydrofuran rings with 80% e.e. Other bis-oxazoline ligands derived from tartaric
CHAPTER 4
acid were screened and the best results were obtained with a 2-naphthyl ligand, which
Electrophilic Additions gave more than 90% e.e. in several cases.
to Carbon-Carbon
Multiple Bonds
Hg(O CCF ) TBDPSO O
2
3 2
TBDPSO OH
cat
68% yield, 86% e.e.
CH C H
CH 3 CH 3 3 2 5
O O O O
N N O O
Ph Ph N N
PhBOX Napth Napth Ref. 114
4.3. Electrophilic Substitution to Carbonyl Groups
4.3.1. Halogenation to Carbonyl Groups
Although the reaction of ketones and other carbonyl compounds with electrophiles
such as bromine leads to substitution rather than addition, the mechanism of the
reaction is closely related to electrophilic additions to alkenes. An enol, enolate, or
enolate equivalent derived from the carbonyl compound is the nucleophile, and the
electrophilic attack by the halogen is analogous to that on alkenes. The reaction is
completed by restoration of the carbonyl bond, rather than by addition of a nucleophile.
The acid- and base-catalyzed halogenation of ketones, which is discussed briefly in
Section 6.4 of Part A, provide the most-studied examples of the reaction from a
mechanistic perspective.
O OH OH O
H + Br 2
R CHCR′ R C CR′ R C CR′ R CCR′
2
2
2
2
Br Br Br
O –OH O – O – O
Br 2
R CHCR′ R 2 C CR′ R C CR′ R CCR′
2
2
2
Br Br Br
The reactions involving bromine or chlorine generate hydrogen halide and are
autocatalytic. Reactions with N-bromosuccinimide or tetrabromocyclohexadienone do
not form any hydrogen bromide and may therefore be preferable reagents in the
case of acid-sensitive compounds. Under some conditions halogenation is faster than
enolization. When this is true, the position of substitution in unsymmetrical ketones
is governed by the relative rates of formation of the isomeric enols. In general,
mixtures are formed with unsymmetrical ketones. The presence of a halogen substituent
114
S. H. Kang and M. Kim, J. Am. Chem. Soc., 125, 4684 (2003).